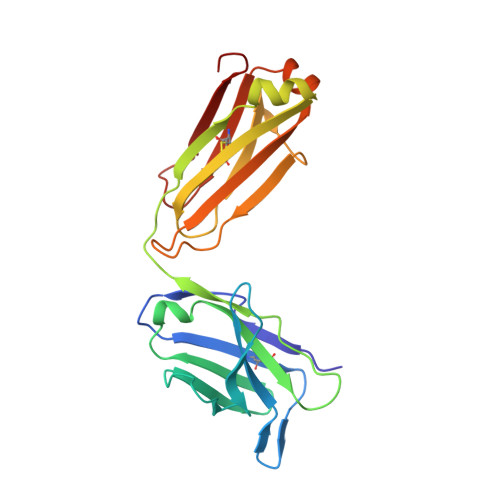
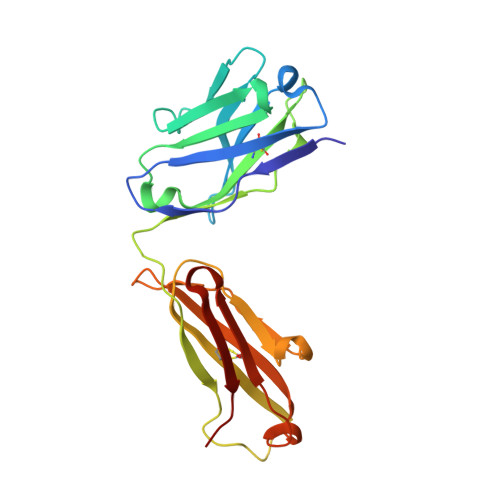
Structural basis of human LRG1 recognition by Magacizumab, a humanized monoclonal antibody with therapeutic potential.
Gutierrez-Fernandez, J., Javaid, F., De Rossi, G., Chudasama, V., Greenwood, J., Moss, S.E., Luecke, H.(2022) Acta Crystallogr D Struct Biol 78: 725-734
- PubMed: 35647920
- DOI: https://doi.org/10.1107/S2059798322004132
- Primary Citation of Related Structures:
7Q4Q - PubMed Abstract:
The formation of new dysfunctional blood vessels is a crucial stage in the development of various conditions such as macular degeneration, diabetes, cardiovascular disease, neurological disease and inflammatory disorders, as well as during tumor growth, eventually contributing to metastasis. An important factor involved in pathogenic angiogenesis is leucine-rich α-2-glycoprotein 1 (LRG1), the antibody blockade of which has been shown to lead to a reduction in both choroidal neovascularization and tumor growth in mouse models. In this work, the structural interactions between the LRG1 epitope and the Fab fragment of Magacizumab, a humanized function-blocking IgG4 against LRG1, are analysed, determining its specific binding mode and the key residues involved in LRG1 recognition. Based on these structural findings, a series of mutations are suggested that could be introduced into Magacizumab to increase its affinity for LRG1, as well as a model of the entire Fab-LRG1 complex that could enlighten new strategies to enhance affinity, consequently leading towards an even more efficient therapeutic.
- Structural Biology and Drug Discovery Group, Centre for Molecular Medicine Norway, Nordic EMBL Partnership, University of Oslo and Oslo University Hospital, 0318 Oslo, Norway.
Organizational Affiliation: