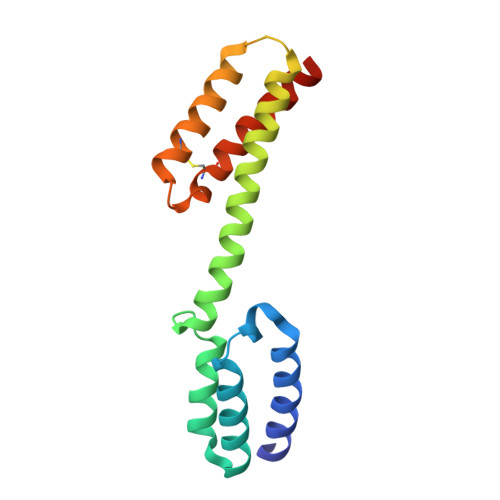
Structural insights into crista junction formation by the Mic60-Mic19 complex.
Bock-Bierbaum, T., Funck, K., Wollweber, F., Lisicki, E., von der Malsburg, K., von der Malsburg, A., Laborenz, J., Noel, J.K., Hessenberger, M., Jungbluth, S., Bernert, C., Kunz, S., Riedel, D., Lilie, H., Jakobs, S., van der Laan, M., Daumke, O.(2022) Sci Adv 8: eabo4946-eabo4946
- PubMed: 36044574
- DOI: https://doi.org/10.1126/sciadv.abo4946
- Primary Citation of Related Structures:
7PUZ, 7PV0, 7PV1 - PubMed Abstract:
Mitochondrial cristae membranes are the oxidative phosphorylation sites in cells. Crista junctions (CJs) form the highly curved neck regions of cristae and are thought to function as selective entry gates into the cristae space. Little is known about how CJs are generated and maintained. We show that the central coiled-coil (CC) domain of the mitochondrial contact site and cristae organizing system subunit Mic60 forms an elongated, bow tie-shaped tetrameric assembly. Mic19 promotes Mic60 tetramerization via a conserved interface between the Mic60 mitofilin and Mic19 CHCH (CC-helix-CC-helix) domains. Dimerization of mitofilin domains exposes a crescent-shaped membrane-binding site with convex curvature tailored to interact with the curved CJ neck. Our study suggests that the Mic60-Mic19 subcomplex traverses CJs as a molecular strut, thereby controlling CJ architecture and function.
- Structural Biology, Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC), Berlin, Germany.
Organizational Affiliation: