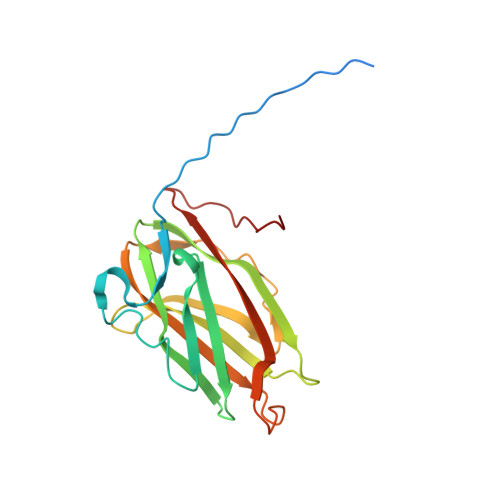
Electron cryo-microscopy reveals the structure of the archaeal thread filament.
Gaines, M.C., Isupov, M.N., Sivabalasarma, S., Haque, R.U., McLaren, M., Mollat, C.L., Tripp, P., Neuhaus, A., Gold, V.A.M., Albers, S.V., Daum, B.(2022) Nat Commun 13: 7411-7411
- PubMed: 36456543
- DOI: https://doi.org/10.1038/s41467-022-34652-4
- Primary Citation of Related Structures:
7PNB - PubMed Abstract:
Pili are filamentous surface extensions that play roles in bacterial and archaeal cellular processes such as adhesion, biofilm formation, motility, cell-cell communication, DNA uptake and horizontal gene transfer. The model archaeaon Sulfolobus acidocaldarius assembles three filaments of the type-IV pilus superfamily (archaella, archaeal adhesion pili and UV-inducible pili), as well as a so-far uncharacterised fourth filament, named "thread". Here, we report on the cryo-EM structure of the archaeal thread. The filament is highly glycosylated and consists of subunits of the protein Saci_0406, arranged in a head-to-tail manner. Saci_0406 displays structural similarity, but low sequence homology, to bacterial type-I pilins. Thread subunits are interconnected via donor strand complementation, a feature reminiscent of bacterial chaperone-usher pili. However, despite these similarities in overall architecture, archaeal threads appear to have evolved independently and are likely assembled by a distinct mechanism.
- Living Systems Institute, University of Exeter, Stocker Road, EX4 4QD, Exeter, UK.
Organizational Affiliation: