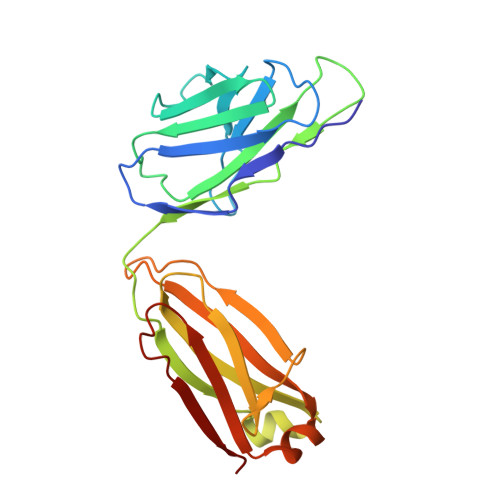
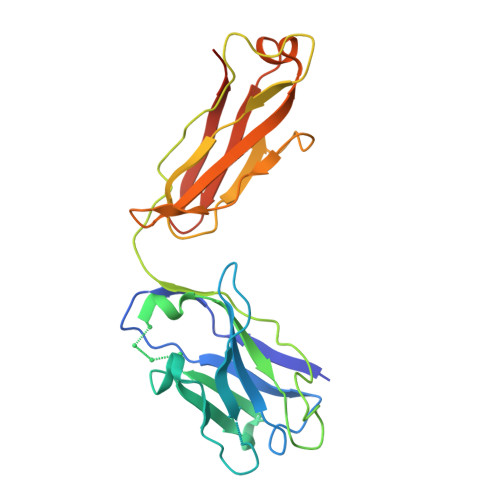
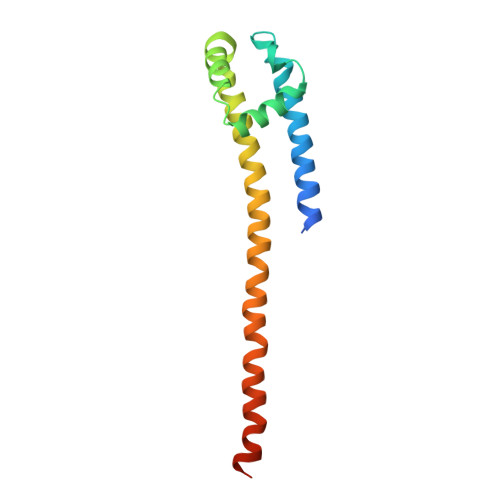
Quaternary structure independent folding of voltage-gated ion channel pore domain subunits.
Arrigoni, C., Lolicato, M., Shaya, D., Rohaim, A., Findeisen, F., Fong, L.K., Colleran, C.M., Dominik, P., Kim, S.S., Schuermann, J.P., DeGrado, W.F., Grabe, M., Kossiakoff, A.A., Minor Jr., D.L.(2022) Nat Struct Mol Biol 29: 537-548
- PubMed: 35655098
- DOI: https://doi.org/10.1038/s41594-022-00775-x
- Primary Citation of Related Structures:
7PG8, 7PGB, 7PGF, 7PGG, 7PGH, 7PGI - PubMed Abstract:
Every voltage-gated ion channel (VGIC) has a pore domain (PD) made from four subunits, each comprising an antiparallel transmembrane helix pair bridged by a loop. The extent to which PD subunit structure requires quaternary interactions is unclear. Here, we present crystal structures of a set of bacterial voltage-gated sodium channel (BacNa V ) 'pore only' proteins that reveal a surprising collection of non-canonical quaternary arrangements in which the PD tertiary structure is maintained. This context-independent structural robustness, supported by molecular dynamics simulations, indicates that VGIC-PD tertiary structure is independent of quaternary interactions. This fold occurs throughout the VGIC superfamily and in diverse transmembrane and soluble proteins. Strikingly, characterization of PD subunit-binding Fabs indicates that non-canonical quaternary PD conformations can occur in full-length VGICs. Together, our data demonstrate that the VGIC-PD is an autonomously folded unit. This property has implications for VGIC biogenesis, understanding functional states, de novo channel design, and VGIC structural origins.
- Cardiovascular Research Institute, University of California, San Francisco, CA, USA.
Organizational Affiliation: