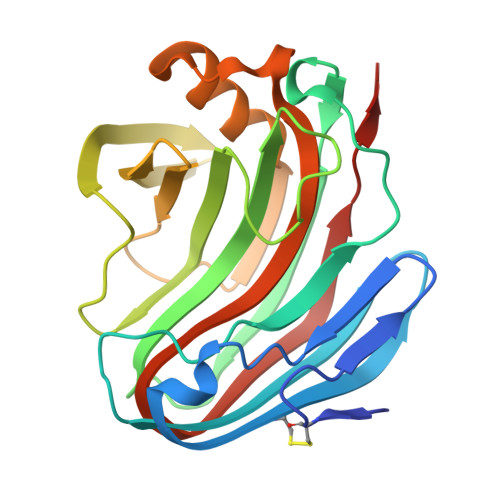
Unusual substrate specificity in GH family 12: structure-function analysis of glucanases Bgh12A and Xgh12B from Aspergillus cervinus, and Egh12 from Thielavia terrestris.
Rykov, S.V., Selimzyanova, A.I., Nikolaeva, A.Y., Lazarenko, V.A., Tsurin, N.V., Akentyev, P.I., Zverlov, V.V., Liebl, W., Schwarz, W.H., Berezina, O.V.(2022) Appl Microbiol Biotechnol 106: 1493-1509
- PubMed: 35129654
- DOI: https://doi.org/10.1007/s00253-022-11811-7
- Primary Citation of Related Structures:
7P1Z - PubMed Abstract:
In this study, we compared the properties and structures of three fungal GH12 enzymes: the strict endoglucanase Bgh12A and the xyloglucanase Xgh12B from Aspergillus cervinus, and the endoglucanase Egh12 from Thielavia terrestris combining activity on linear β-glucan and branched xyloglucan. Egh12 from T. terrestris was produced in Pichia pastoris, purified, and characterized as a thermostable enzyme with maximal activity at 70 ºC and a half-life time of 138 min at 65 °C. We for the first time demonstrated that the GH12 endoglucanases Egh12 and Bgh12A, but not the strict xyloglucanase Xgh12B, hydrolyzed (1,3)-β-linkages in (1,3;1,4)-β-D-glucooligosaccharides and had transglycosylase activity on (1,3)-β-D-glucooligosaccharides. Phylogenetic analysis indicated that Egh12 from T. terrestris and Bgh12A from A. cervinus are more related than Bgh12A and Xgh12B isolated from one strain. The X-ray structure of Bgh12A was determined with 2.17 Å resolution and compared with 3D-homology models of Egh12 and Xgh12B. The enzymes have a β-jelly roll structure with a catalytic cleft running across the protein. Comparative analysis and a docking study demonstrated the importance of endoglucanase-specific loop 1 partly covering the catalytic cleft for correct placement of the linear substrates. Variability in substrate specificity between the GH12 endoglucanases is determined by non-conservative residues in structural loops framing the catalytic cleft. A residue responsible for the thermostability of Egh12 was predicted. The key structural elements and residues described in this study may serve as potential targets for modification aimed at the improvement of enzymatic properties. KEY POINTS: • Thermostable endoglucanase Egh12 from T. terrestris was produced in P. pastoris, purified, and characterized • The X-ray structure of GH12 endoglucanase Bgh12A from A. cervinus was resolved • GH12 endoglucanases, but not GH12 xyloglucanases, hydrolyze (1,3)-β-linkages in (1,3;1,4)-β-D-glucooligosaccharides.
- National Research Center «Kurchatov Institute» - GOSNIIGENETIKA, Kurchatov Genomic Center, 1-st Dorozhniy pr. 1, 117545, Moscow, Russian Federation.
Organizational Affiliation: