A New Class of Bi- and Trifunctional Sugar Oximes as Antidotes against Organophosphorus Poisoning.
Da Silva, O., Probst, N., Landry, C., Hanak, A.S., Warnault, P., Coisne, C., Calas, A.G., Gosselet, F., Courageux, C., Gastellier, A.J., Trancart, M., Baati, R., Dehouck, M.P., Jean, L., Nachon, F., Renard, P.Y., Dias, J.(2022) J Med Chem 65: 4649-4666
- PubMed: 35255209
- DOI: https://doi.org/10.1021/acs.jmedchem.1c01748
- Primary Citation of Related Structures:
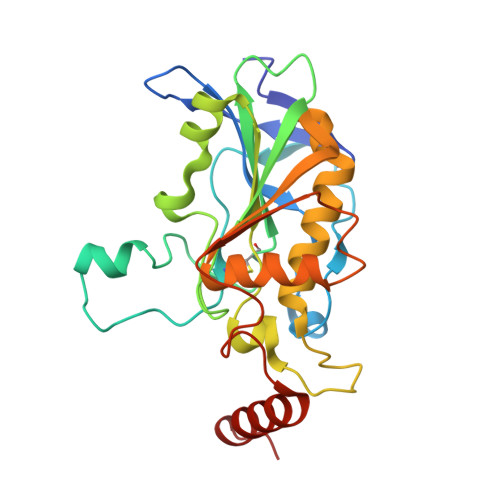
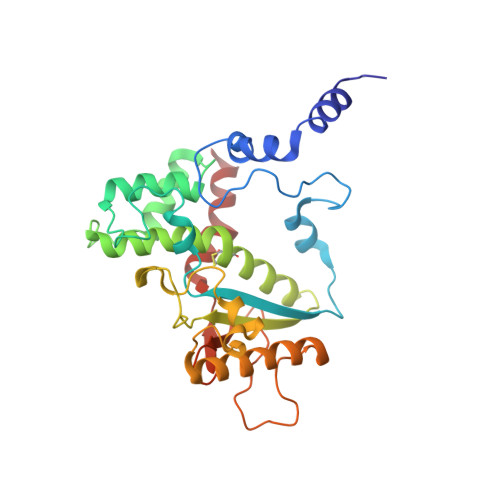
7P1N, 7P1P - PubMed Abstract:
Recent events demonstrated that organophosphorus nerve agents are a serious threat for civilian and military populations. The current therapy includes a pyridinium aldoxime reactivator to restore the enzymatic activity of acetylcholinesterase located in the central nervous system and neuro-muscular junctions. One major drawback of these charged acetylcholinesterase reactivators is their poor ability to cross the blood-brain barrier. In this study, we propose to evaluate glucoconjugated oximes devoid of permanent charge as potential central nervous system reactivators. We determined their in vitro reactivation efficacy on inhibited human acetylcholinesterase, the crystal structure of two compounds in complex with the enzyme, their protective index on intoxicated mice, and their pharmacokinetics. We then evaluated their endothelial permeability coefficients with a human in vitro model. This study shed light on the structural restrains of new sugar oximes designed to reach the central nervous system through the glucose transporter located at the blood-brain barrier.
- Département de Toxicologie et Risques Chimiques, Institut de Recherche Biomédicale Des Armées, F-91220 Brétigny-Sur-Orge, France.
Organizational Affiliation: