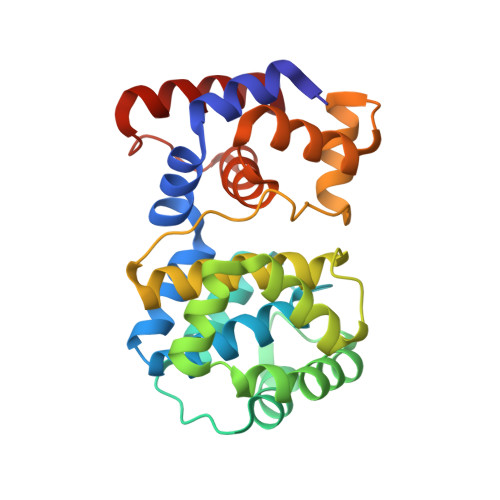
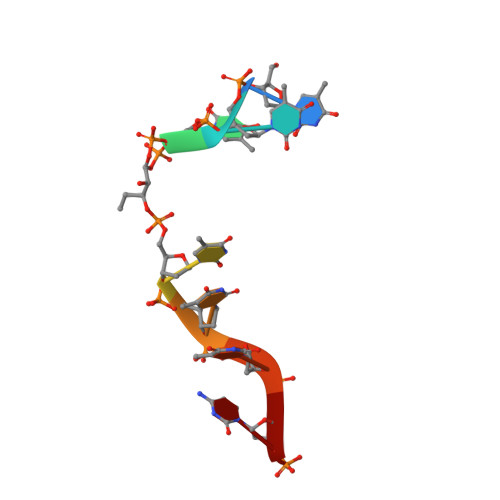
Structural and functional determinants of the archaeal 8-oxoguanine-DNA glycosylase AGOG for DNA damage recognition and processing.
Franck, C., Stephane, G., Julien, C., Virginie, G., Martine, G., Norbert, G., Fabrice, C., Didier, F., Josef, S.M., Bertrand, C.(2022) Nucleic Acids Res 50: 11072-11092
- PubMed: 36300625
- DOI: https://doi.org/10.1093/nar/gkac932
- Primary Citation of Related Structures:
7OLB, 7OLI, 7OU3, 7OUE, 7OY7, 7P0W, 7P8L, 7P9Z - PubMed Abstract:
8-Oxoguanine (GO) is a major purine oxidation product in DNA. Because of its highly mutagenic properties, GO absolutely must be eliminated from DNA. To do this, aerobic and anaerobic organisms from the three kingdoms of life have evolved repair mechanisms to prevent its deleterious effect on genetic integrity. The major way to remove GO is the base excision repair pathway, usually initiated by a GO-DNA glycosylase. First identified in bacteria (Fpg) and eukaryotes (OGG1), GO-DNA glycosylases were more recently identified in archaea (OGG2 and AGOG). AGOG is the less documented enzyme and its mode of damage recognition and removing remains to be clarified at the molecular and atomic levels. This study presents a complete structural characterisation of apo AGOGs from Pyrococcus abyssi (Pab) and Thermococcus gammatolerans (Tga) and the first structure of Pab-AGOG bound to lesion-containing single- or double-stranded DNA. By combining X-ray structure analysis, site directed mutagenesis and biochemistry experiments, we identified key amino acid residues of AGOGs responsible for the specific recognition of the lesion and the base opposite the lesion and for catalysis. Moreover, a unique binding mode of GO, involving double base flipping, never observed for any other DNA glycosylases, is revealed. In addition to unravelling the properties of AGOGs, our study, through comparative biochemical and structural analysis, offers new insights into the evolutionary plasticity of DNA glycosylases across all three kingdoms of life.
- Centre de Biophysique Moléculaire (CBM), UPR4301 CNRS, Université d'Orléans, CS 80054, rue Charles Sadron, F-45071 Orléans cedex 02, France.
Organizational Affiliation: