SAKe: Computationally Designed Modular Protein Building Blocks for Macromolecular Assemblies
Wouters, S.M.L., Clarke, D.E., Noguchi, H., Velpula, G., Voet, A.R.D., De Feyter, S.To be published.
Experimental Data Snapshot
Starting Model: in silico
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| SAKe6AE | 310 | synthetic construct | Mutation(s): 0 | 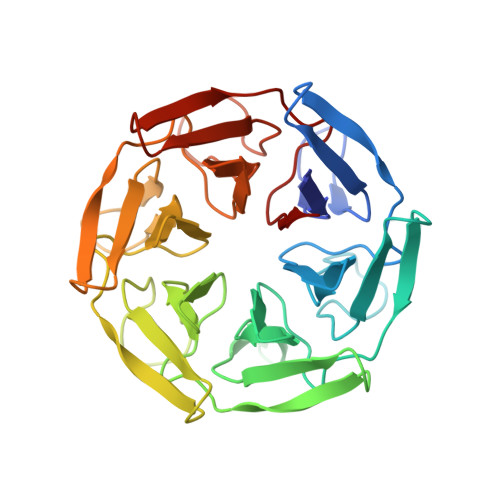 | |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| SO4 Query on SO4 | B [auth A] C [auth A] D [auth A] E [auth A] F [auth A] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 82.767 | α = 90 |
| b = 82.767 | β = 90 |
| c = 90.023 | γ = 120 |
| Software Name | Purpose |
|---|---|
| XDS | data reduction |
| Aimless | data scaling |
| PHASER | phasing |
| PHENIX | refinement |
| PDB_EXTRACT | data extraction |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Research Foundation - Flanders (FWO) | Belgium | 1S89918N |
| Research Foundation - Flanders (FWO) | Belgium | G0F9316N |
| Research Foundation - Flanders (FWO) | Belgium | G051917N |