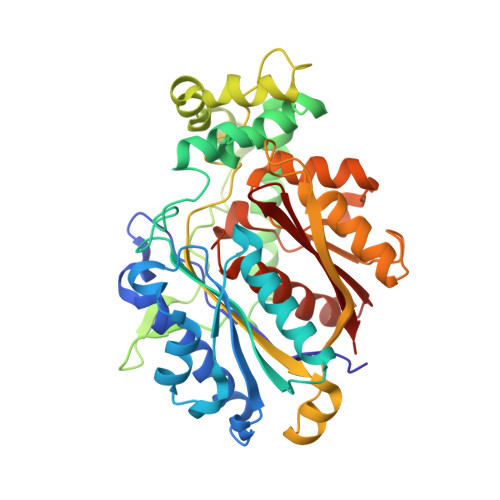
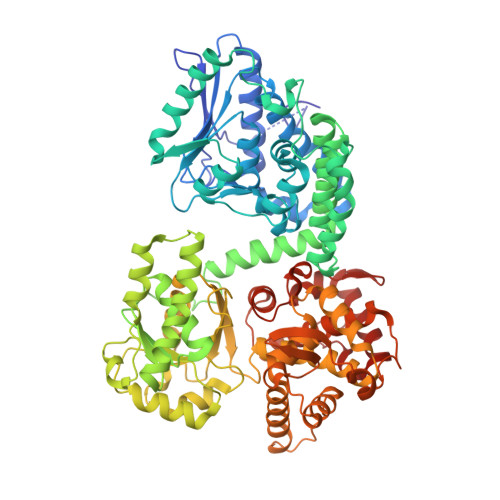
Substrate specificity and conformational flexibility properties of the Mycobacterium tuberculosis beta-oxidation trifunctional enzyme.
Dalwani, S., Lampela, O., Leprovost, P., Schmitz, W., Juffer, A.H., Wierenga, R.K., Venkatesan, R.(2021) J Struct Biol 213: 107776-107776
- PubMed: 34371166
- DOI: https://doi.org/10.1016/j.jsb.2021.107776
- Primary Citation of Related Structures:
7O1G, 7O1I, 7O1J, 7O1K, 7O1L, 7O1M, 7O4Q, 7O4R, 7O4S, 7O4T, 7O4U, 7O4V - PubMed Abstract:
The Mycobacterium tuberculosis trifunctional enzyme (MtTFE) is an α 2 β 2 tetrameric enzyme. The α-chain harbors the 2E-enoyl-CoA hydratase (ECH) and 3S-hydroxyacyl-CoA dehydrogenase (HAD) activities and the β-chain provides the 3-ketoacyl-CoA thiolase (KAT) activity. Enzyme kinetic data reported here show that medium and long chain enoyl-CoA molecules are preferred substrates for MtTFE. Modelling studies indicate how the linear medium and long acyl chains of these substrates can bind to each of the active sites. In addition, crystallographic binding studies have identified three new CoA binding sites which are different from the previously known CoA binding sites of the three TFE active sites. Structure comparisons provide new insights into the properties of ECH, HAD and KAT active sites of MtTFE. The interactions of the adenine moiety of CoA with loop-2 of the ECH active site cause a conformational change of this loop by which a competent ECH active site is formed. The NAD + binding domain (domain C) of the HAD part of MtTFE has only a few interactions with the rest of the complex and adopts a range of open conformations, whereas the A-domain of the ECH part is rigidly fixed with respect to the HAD part. Two loops, the CB1-CA1 region and the catalytic CB4-CB5 loop, near the thiolase active site and the thiolase dimer interface, have high B-factors. Structure comparisons suggest that a competent and stable thiolase dimer is formed only when complexed with the α-chains, highlighting the importance of the assembly for the proper functioning of the complex.
- Faculty of Biochemistry and Molecular Medicine, University of Oulu, Oulu, Finland.
Organizational Affiliation: