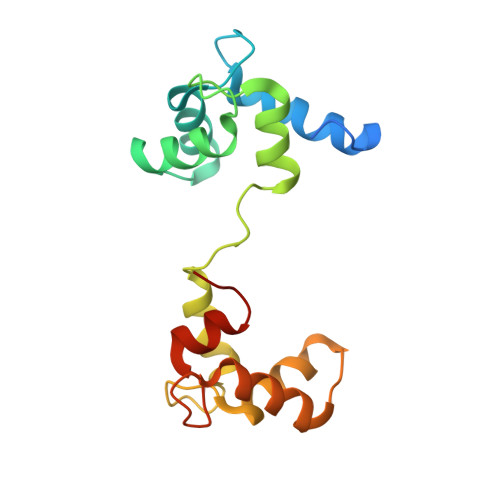
Calmodulin extracts the Ras family protein RalA from lipid bilayers by engagement with two membrane-targeting motifs.
Chamberlain, S.G., Gohlke, A., Shafiq, A., Squires, I.J., Owen, D., Mott, H.R.(2021) Proc Natl Acad Sci U S A 118
- PubMed: 34480001
- DOI: https://doi.org/10.1073/pnas.2104219118
- Primary Citation of Related Structures:
7NQC - PubMed Abstract:
RalA is a small GTPase and a member of the Ras family. This molecular switch is activated downstream of Ras and is widely implicated in tumor formation and growth. Previous work has shown that the ubiquitous Ca 2+ -sensor calmodulin (CaM) binds to small GTPases such as RalA and K-Ras4B, but a lack of structural information has obscured the functional consequences of these interactions. Here, we have investigated the binding of CaM to RalA and found that CaM interacts exclusively with the C terminus of RalA, which is lipidated with a prenyl group in vivo to aid membrane attachment. Biophysical and structural analyses show that the two RalA membrane-targeting motifs (the prenyl anchor and the polybasic motif) are engaged by distinct lobes of CaM and that CaM binding leads to removal of RalA from its membrane environment. The structure of this complex, along with a biophysical investigation into membrane removal, provides a framework with which to understand how CaM regulates the function of RalA and sheds light on the interaction of CaM with other small GTPases, including K-Ras4B.
- Department of Biochemistry, University of Cambridge, Cambridge CB2 1GA, United Kingdom.
Organizational Affiliation: