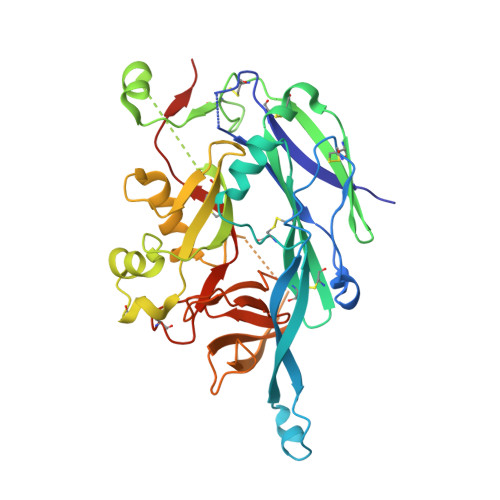
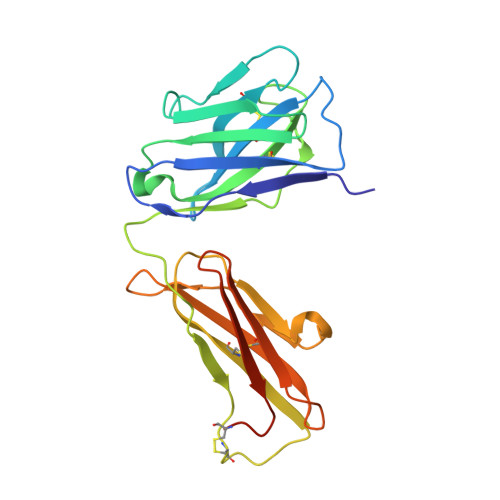
Structural Basis for a Neutralizing Antibody Response Elicited by a Recombinant Hantaan Virus Gn Immunogen.
Rissanen, I., Krumm, S.A., Stass, R., Whitaker, A., Voss, J.E., Bruce, E.A., Rothenberger, S., Kunz, S., Burton, D.R., Huiskonen, J.T., Botten, J.W., Bowden, T.A., Doores, K.J.(2021) mBio 12: e0253120-e0253120
- PubMed: 34225492
- DOI: https://doi.org/10.1128/mBio.02531-20
- Primary Citation of Related Structures:
7NKS, 7NRH, 7O9S - PubMed Abstract:
Hantaviruses are a group of emerging pathogens capable of causing severe disease upon zoonotic transmission to humans. The mature hantavirus surface presents higher-order tetrameric assemblies of two glycoproteins, Gn and Gc, which are responsible for negotiating host cell entry and constitute key therapeutic targets. Here, we demonstrate that recombinantly derived Gn from Hantaan virus (HTNV) elicits a neutralizing antibody response (serum dilution that inhibits 50% infection [ID 50 ], 1:200 to 1:850) in an animal model. Using antigen-specific B cell sorting, we isolated monoclonal antibodies (mAbs) exhibiting neutralizing and non-neutralizing activity, termed mAb HTN-Gn1 and mAb nnHTN-Gn2, respectively. Crystallographic analysis reveals that these mAbs target spatially distinct epitopes at disparate sites of the N-terminal region of the HTNV Gn ectodomain. Epitope mapping onto a model of the higher order (Gn-Gc) 4 spike supports the immune accessibility of the mAb HTN-Gn1 epitope, a hypothesis confirmed by electron cryo-tomography of the antibody with virus-like particles. These data define natively exposed regions of the hantaviral Gn that can be targeted in immunogen design. IMPORTANCE The spillover of pathogenic hantaviruses from rodent reservoirs into the human population poses a continued threat to human health. Here, we show that a recombinant form of the Hantaan virus (HTNV) surface-displayed glycoprotein, Gn, elicits a neutralizing antibody response in rabbits. We isolated a neutralizing (HTN-Gn1) and a non-neutralizing (nnHTN-Gn2) monoclonal antibody and provide the first molecular-level insights into how the Gn glycoprotein may be targeted by the antibody-mediated immune response. These findings may guide rational vaccine design approaches focused on targeting the hantavirus glycoprotein envelope.
- Division of Structural Biology, Wellcome Centre for Human Genetics, grid.4991.5University of Oxford, Oxford, United Kingdom.
Organizational Affiliation: