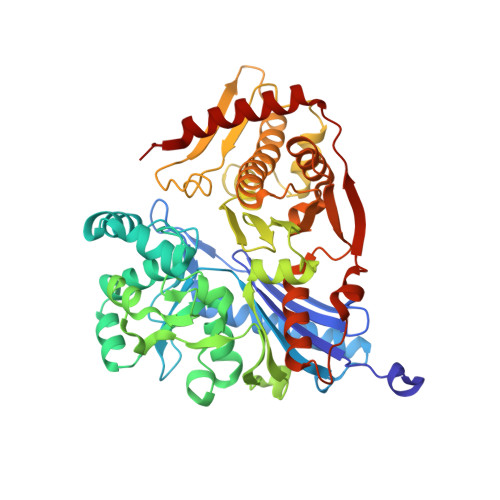
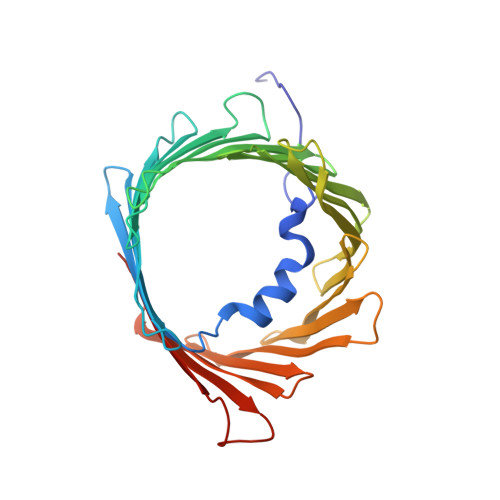
In-cell structures of conserved supramolecular protein arrays at the mitochondria-cytoskeleton interface in mammalian sperm.
Leung, M.R., Zenezini Chiozzi, R., Roelofs, M.C., Hevler, J.F., Ravi, R.T., Maitan, P., Zhang, M., Henning, H., Bromfield, E.G., Howes, S.C., Gadella, B.M., Heck, A.J.R., Zeev-Ben-Mordehai, T.(2021) Proc Natl Acad Sci U S A 118
- PubMed: 34737233
- DOI: https://doi.org/10.1073/pnas.2110996118
- Primary Citation of Related Structures:
7NIE - PubMed Abstract:
Mitochondria-cytoskeleton interactions modulate cellular physiology by regulating mitochondrial transport, positioning, and immobilization. However, there is very little structural information defining mitochondria-cytoskeleton interfaces in any cell type. Here, we use cryofocused ion beam milling-enabled cryoelectron tomography to image mammalian sperm, where mitochondria wrap around the flagellar cytoskeleton. We find that mitochondria are tethered to their neighbors through intermitochondrial linkers and are anchored to the cytoskeleton through ordered arrays on the outer mitochondrial membrane. We use subtomogram averaging to resolve in-cell structures of these arrays from three mammalian species, revealing they are conserved across species despite variations in mitochondrial dimensions and cristae organization. We find that the arrays consist of boat-shaped particles anchored on a network of membrane pores whose arrangement and dimensions are consistent with voltage-dependent anion channels. Proteomics and in-cell cross-linking mass spectrometry suggest that the conserved arrays are composed of glycerol kinase-like proteins. Ordered supramolecular assemblies may serve to stabilize similar contact sites in other cell types in which mitochondria need to be immobilized in specific subcellular environments, such as in muscles and neurons.
- Bijvoet Centre for Biomolecular Research, Utrecht University, Utrecht 3584 CH, The Netherlands.
Organizational Affiliation: