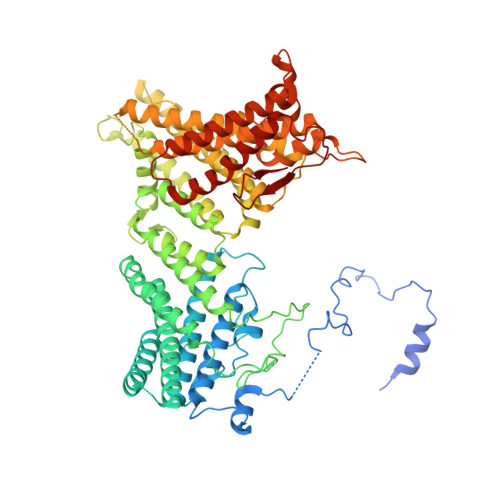
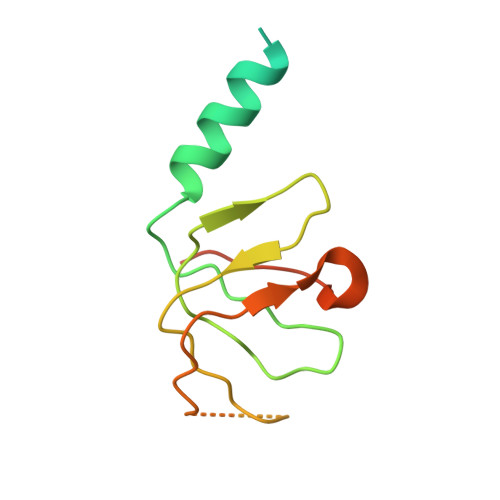
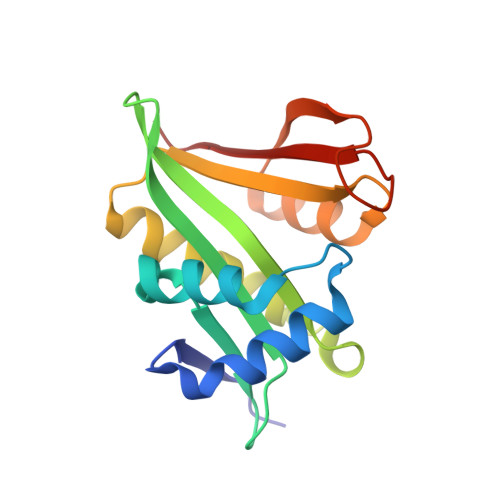
Molecular role of NAA38 in thermostability and catalytic activity of the human NatC N-terminal acetyltransferase.
Deng, S., Gardner, S.M., Gottlieb, L., Pan, B., Petersson, E.J., Marmorstein, R.(2023) Structure 31: 166-173.e4
- PubMed: 36638802
- DOI: https://doi.org/10.1016/j.str.2022.12.008
- Primary Citation of Related Structures:
7MX2, 7RB3 - PubMed Abstract:
N-terminal acetylation occurs on over 80% of human proteins and is catalyzed by a family of N-terminal acetyltransferases (NATs). All NATs contain a small catalytic subunit, while some also contain a large auxiliary subunit that facilitates catalysis and ribosome targeting for co-translational acetylation. NatC is one of the major NATs containing an NAA30 catalytic subunit, but uniquely contains two auxiliary subunits, large NAA35 and small NAA38. Here, we report the cryo-EM structures of human NatC (hNatC) complexes with and without NAA38, together with biochemical studies, to reveal that NAA38 increases the thermostability and broadens the substrate-specificity profile of NatC by ordering an N-terminal segment of NAA35 and reorienting an NAA30 N-terminal peptide binding loop for optimal catalysis, respectively. We also note important differences in engagement with a stabilizing inositol hexaphosphate molecule between human and yeast NatC. These studies provide new insights for the function and evolution of the NatC complex.
- Department of Chemistry, University of Pennsylvania, 231 South 34(th) Street, Philadelphia, PA 19104, USA; Abramson Family Cancer Research Institute, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA.
Organizational Affiliation: