The structure of succinyl-CoA synthetase bound to the succinyl-phosphate intermediate clarifies the catalytic mechanism of ATP-citrate lyase
Huang, J., Fraser, M.E.(2022) Acta Crystallogr F Struct Biol Commun 78: 363-370
Experimental Data Snapshot
Starting Model: experimental
View more details
(2022) Acta Crystallogr F Struct Biol Commun 78: 363-370
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Succinate--CoA ligase [ADP/GDP-forming] subunit alpha, mitochondrial | 315 | Homo sapiens | Mutation(s): 1 Gene Names: SUCLG1 EC: 6.2.1.4 (PDB Primary Data), 6.2.1.5 (PDB Primary Data), 6.2.1.9 (UniProt), 6.2.1 (UniProt) | 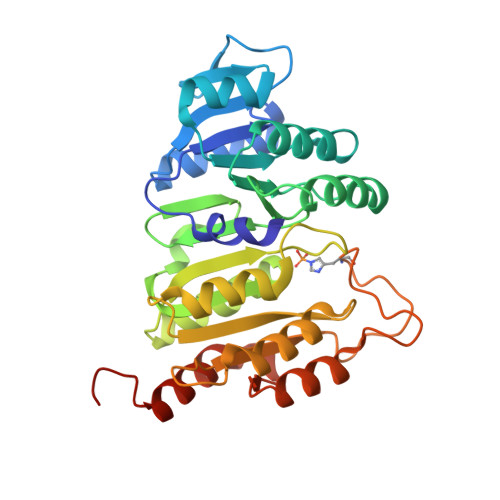 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P53597 (Homo sapiens) Explore P53597 Go to UniProtKB: P53597 | |||||
PHAROS: P53597 GTEx: ENSG00000163541 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P53597 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Succinate--CoA ligase [GDP-forming] subunit beta, mitochondrial | 395 | Homo sapiens | Mutation(s): 0 Gene Names: SUCLG2 EC: 6.2.1.4 (PDB Primary Data), 6.2.1 (UniProt) | 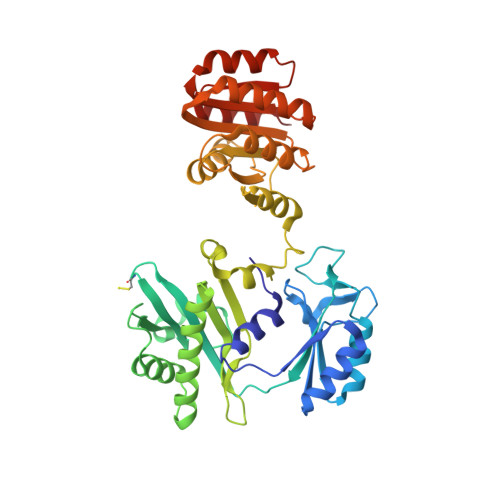 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q96I99 (Homo sapiens) Explore Q96I99 Go to UniProtKB: Q96I99 | |||||
PHAROS: Q96I99 GTEx: ENSG00000172340 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q96I99 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| COA (Subject of Investigation/LOI) Query on COA | C [auth A] | COENZYME A C21 H36 N7 O16 P3 S RGJOEKWQDUBAIZ-IBOSZNHHSA-N |  | ||
| GOL Query on GOL | D [auth A], E [auth A], G [auth A] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| EDO Query on EDO | F [auth A], H [auth A] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| Modified Residues 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| NEP Query on NEP | A | L-PEPTIDE LINKING | C6 H10 N3 O5 P |  | HIS |
| CSS Query on CSS | B | L-PEPTIDE LINKING | C3 H7 N O2 S2 |  | CYS |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 87.82 | α = 90 |
| b = 82.927 | β = 103.47 |
| c = 48.828 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| xia2 | data scaling |
| PDB_EXTRACT | data extraction |
| Coot | model building |
| PHASER | phasing |
| DIALS | data reduction |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Natural Sciences and Engineering Research Council (NSERC, Canada) | Canada | 04815-2019 |