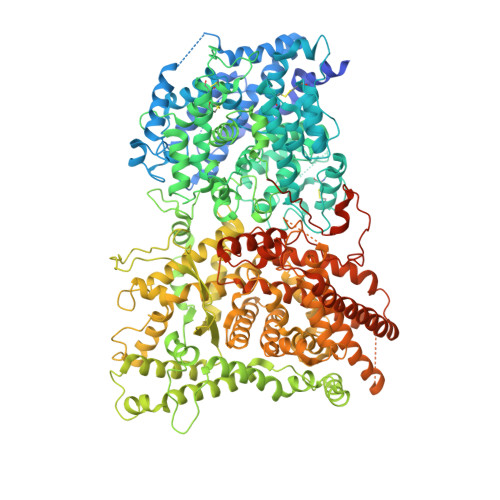
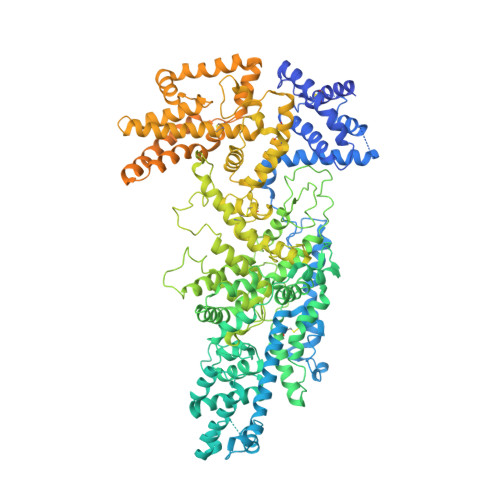
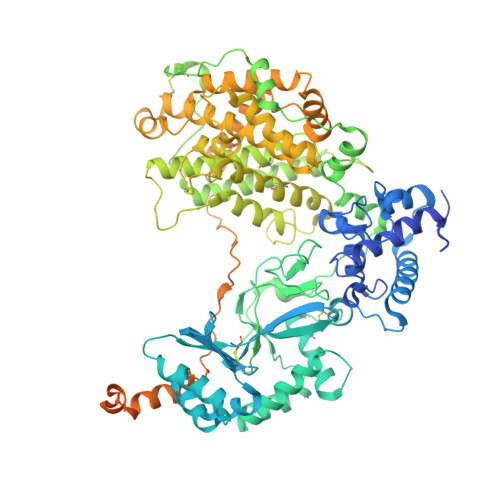
Native structure of the RhopH complex, a key determinant of malaria parasite nutrient acquisition.
Ho, C.M., Jih, J., Lai, M., Li, X., Goldberg, D.E., Beck, J.R., Zhou, Z.H.(2021) Proc Natl Acad Sci U S A 118
- PubMed: 34446549
- DOI: https://doi.org/10.1073/pnas.2100514118
- Primary Citation of Related Structures:
7MRW - PubMed Abstract:
The RhopH complex is implicated in malaria parasites' ability to invade and create new permeability pathways in host erythrocytes, but its mechanisms remain poorly understood. Here, we enrich the endogenous RhopH complex in a native soluble form, comprising RhopH2, CLAG3.1, and RhopH3, directly from parasite cell lysates and determine its atomic structure using cryo-electron microscopy (cryo-EM), mass spectrometry, and the cryoID program. CLAG3.1 is positioned between RhopH2 and RhopH3, which both share substantial binding interfaces with CLAG3.1 but make minimal contacts with each other. The forces stabilizing individual subunits include 13 intramolecular disulfide bonds. Notably, CLAG3.1 residues 1210 to 1223, previously predicted to constitute a transmembrane helix, are embedded within a helical bundle formed by residues 979 to 1289 near the C terminus of CLAG3.1. Buried in the core of the RhopH complex and largely shielded from solvent, insertion of this putative transmembrane helix into the erythrocyte membrane would likely require a large conformational rearrangement. Given the unusually high disulfide content of the complex, it is possible that such a rearrangement could be initiated by the breakage of allosteric disulfide bonds, potentially triggered by interactions at the erythrocyte membrane. This first direct observation of an exported Plasmodium falciparum transmembrane protein-in a soluble, trafficking state and with atomic details of buried putative membrane-insertion helices-offers insights into the assembly and trafficking of RhopH and other parasite-derived complexes to the erythrocyte membrane. Our study demonstrates the potential the endogenous structural proteomics approach holds for elucidating the molecular mechanisms of hard-to-isolate complexes in their native, functional forms.
- Department of Microbiology & Immunology, Columbia University, New York, NY 10032; chi-min.ho@columbia.edu Hong.Zhou@UCLA.edu.
Organizational Affiliation: