Ribonucleotide Reductase
Palowitch, G.M., Boal, A.K.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Ribonucleoside-diphosphate reductase | 337 | Aerococcus sp. Group 1 | Mutation(s): 0 Gene Names: HMPREF9243_0731 EC: 1.17.4.1 | 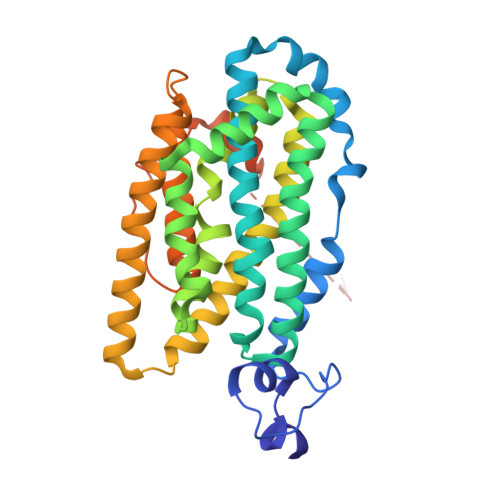 | |
UniProt | |||||
Find proteins for A0ACD6B8K0 (Aerococcus urinae (strain CCUG 59500 / ACS-120-V-Col10a)) Explore A0ACD6B8K0 Go to UniProtKB: A0ACD6B8K0 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A0ACD6B8K0 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Protein NrdI | E, F [auth G], G [auth F], H | 142 | Aerococcus urinae | Mutation(s): 0 Gene Names: nrdI, CYJ29_07405, DBT54_07500 | 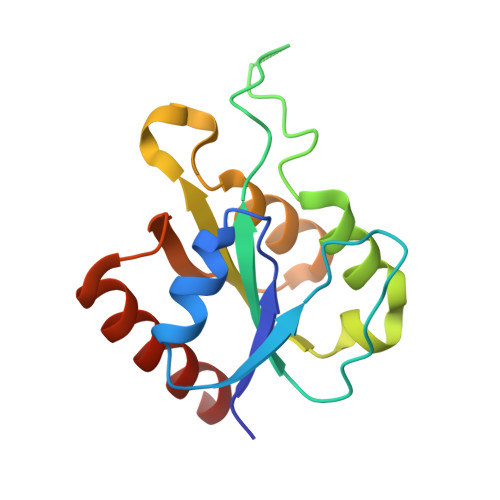 |
UniProt | |||||
Find proteins for A0ACD6B8K1 (Aerococcus urinae (strain CCUG 59500 / ACS-120-V-Col10a)) Explore A0ACD6B8K1 Go to UniProtKB: A0ACD6B8K1 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A0ACD6B8K1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 5 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| FMN (Subject of Investigation/LOI) Query on FMN | BA [auth E], DA [auth G], FA [auth F], HA [auth H] | FLAVIN MONONUCLEOTIDE C17 H21 N4 O9 P FVTCRASFADXXNN-SCRDCRAPSA-N |  | ||
| TRS Query on TRS | S [auth C] | 2-AMINO-2-HYDROXYMETHYL-PROPANE-1,3-DIOL C4 H12 N O3 LENZDBCJOHFCAS-UHFFFAOYSA-O |  | ||
| GOL Query on GOL | AA [auth D] K [auth A] N [auth B] O [auth B] R [auth C] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| CU1 (Subject of Investigation/LOI) Query on CU1 | I [auth A] J [auth A] L [auth B] M [auth B] P [auth C] | COPPER (I) ION Cu VMQMZMRVKUZKQL-UHFFFAOYSA-N |  | ||
| CA Query on CA | CA [auth E] EA [auth G] GA [auth F] IA [auth H] U [auth C] | CALCIUM ION Ca BHPQYMZQTOCNFJ-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 77.398 | α = 90 |
| b = 104.958 | β = 100.01 |
| c = 138.067 | γ = 90 |
| Software Name | Purpose |
|---|---|
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| PHASER | phasing |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | -- |