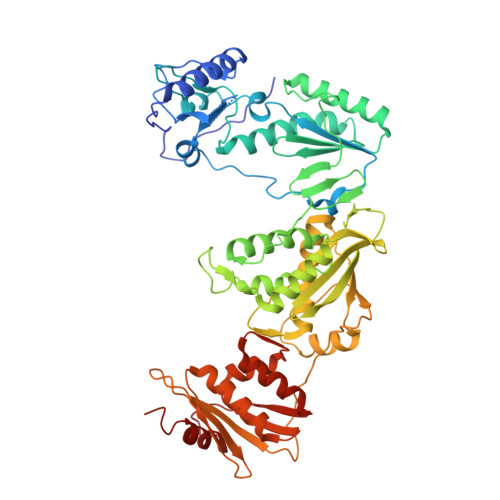
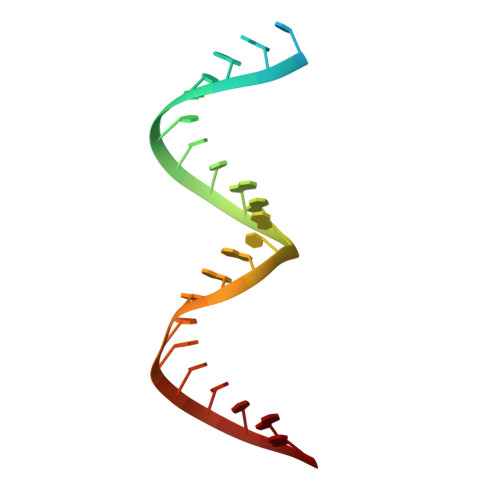
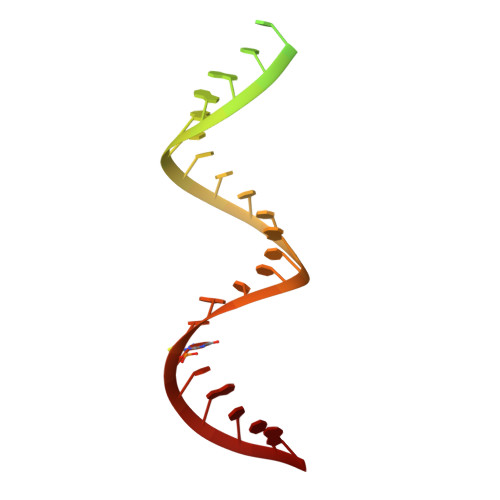
High-resolution view of HIV-1 reverse transcriptase initiation complexes and inhibition by NNRTI drugs.
Ha, B., Larsen, K.P., Zhang, J., Fu, Z., Montabana, E., Jackson, L.N., Chen, D.H., Puglisi, E.V.(2021) Nat Commun 12: 2500-2500
- PubMed: 33947853
- DOI: https://doi.org/10.1038/s41467-021-22628-9
- Primary Citation of Related Structures:
7KJV, 7KJW, 7KJX - PubMed Abstract:
Reverse transcription of the HIV-1 viral RNA genome (vRNA) is an integral step in virus replication. Upon viral entry, HIV-1 reverse transcriptase (RT) initiates from a host tRNA Lys 3 primer bound to the vRNA genome and is the target of key antivirals, such as non-nucleoside reverse transcriptase inhibitors (NNRTIs). Initiation proceeds slowly with discrete pausing events along the vRNA template. Despite prior medium-resolution structural characterization of reverse transcriptase initiation complexes (RTICs), higher-resolution structures of the RTIC are needed to understand the molecular mechanisms that underlie initiation. Here we report cryo-EM structures of the core RTIC, RTIC-nevirapine, and RTIC-efavirenz complexes at 2.8, 3.1, and 2.9 Å, respectively. In combination with biochemical studies, these data suggest a basis for rapid dissociation kinetics of RT from the vRNA-tRNA Lys 3 initiation complex and reveal a specific structural mechanism of nucleic acid conformational stabilization during initiation. Finally, our results show that NNRTIs inhibit the RTIC and exacerbate discrete pausing during early reverse transcription.
- Department of Structural Biology, Stanford University School of Medicine, Stanford, CA, USA.
Organizational Affiliation: