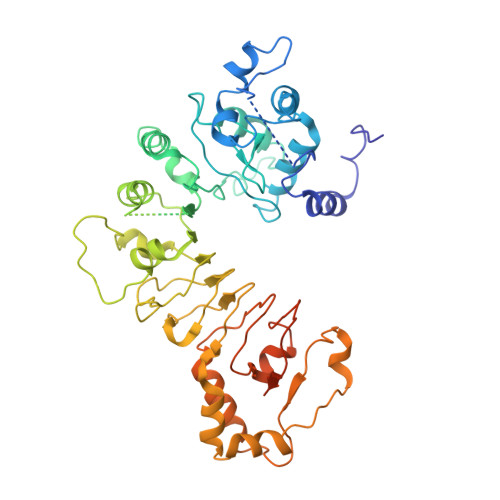
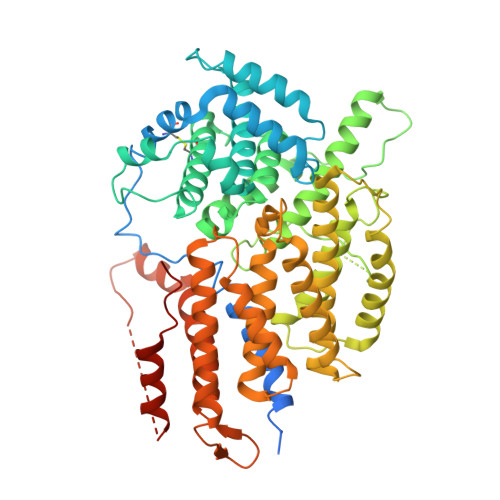
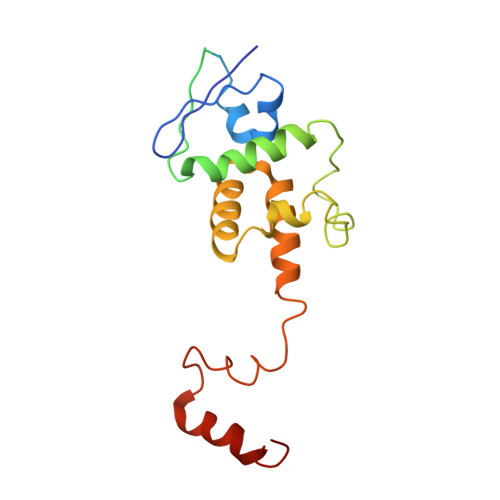
Structural and dynamic mechanisms of CBF3-guided centromeric nucleosome formation.
Guan, R., Lian, T., Zhou, B.R., He, E., Wu, C., Singleton, M., Bai, Y.(2021) Nat Commun 12: 1763-1763
- PubMed: 33741944
- DOI: https://doi.org/10.1038/s41467-021-21985-9
- Primary Citation of Related Structures:
7K78, 7K79, 7K7G - PubMed Abstract:
Accurate chromosome segregation relies on the specific centromeric nucleosome-kinetochore interface. In budding yeast, the centromere CBF3 complex guides the deposition of CENP-A, an H3 variant, to form the centromeric nucleosome in a DNA sequence-dependent manner. Here, we determine the structures of the centromeric nucleosome containing the native CEN3 DNA and the CBF3core bound to the canonical nucleosome containing an engineered CEN3 DNA. The centromeric nucleosome core structure contains 115 base pair DNA including a CCG motif. The CBF3core specifically recognizes the nucleosomal CCG motif through the Gal4 domain while allosterically altering the DNA conformation. Cryo-EM, modeling, and mutational studies reveal that the CBF3core forms dynamic interactions with core histones H2B and CENP-A in the CEN3 nucleosome. Our results provide insights into the structure of the budding yeast centromeric nucleosome and the mechanism of its assembly, which have implications for analogous processes of human centromeric nucleosome formation.
- Laboratory of Biochemistry and Molecular Biology, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Organizational Affiliation: