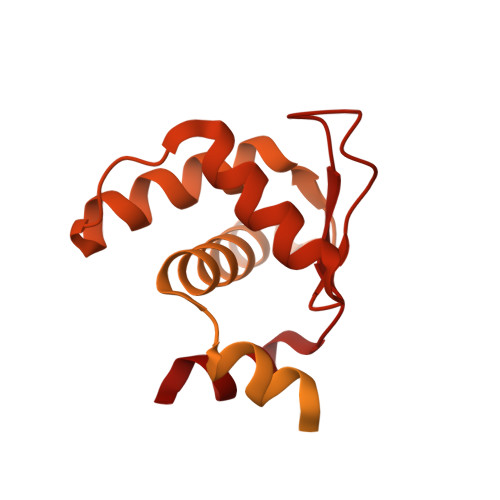
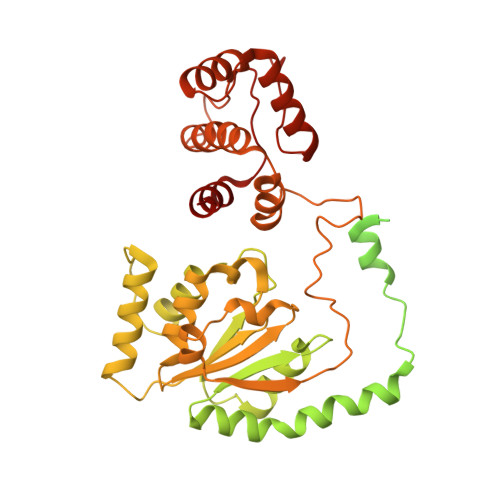
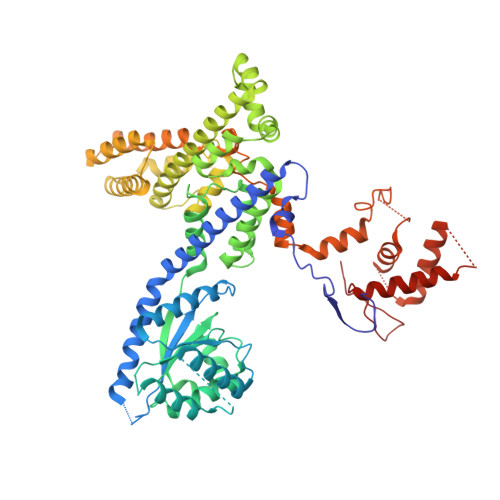
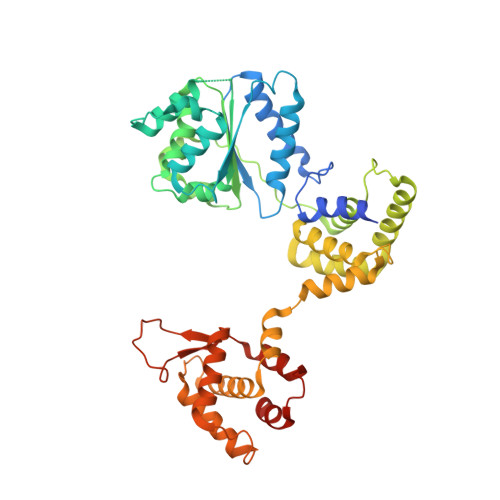
The dynamic nature of the human Origin Recognition Complex revealed through five cryoEM structures.
Jaremko, M.J., On, K.F., Thomas, D.R., Stillman, B., Joshua-Tor, L.(2020) Elife 9
- PubMed: 32808929
- DOI: https://doi.org/10.7554/eLife.58622
- Primary Citation of Related Structures:
7JPO, 7JPP, 7JPQ, 7JPR, 7JPS - PubMed Abstract:
Genome replication is initiated from specific origin sites established by dynamic events. The Origin Recognition Complex (ORC) is necessary for orchestrating the initiation process by binding to origin DNA, recruiting CDC6, and assembling the MCM replicative helicase on DNA. Here we report five cryoEM structures of the human ORC (HsORC) that illustrate the native flexibility of the complex. The absence of ORC1 revealed a compact, stable complex of ORC2-5. Introduction of ORC1 opens the complex into several dynamic conformations. Two structures revealed dynamic movements of the ORC1 AAA+ and ORC2 winged-helix domains that likely impact DNA incorporation into the ORC core. Additional twist and pinch motions were observed in an open ORC conformation revealing a hinge at the ORC5·ORC3 interface that may facilitate ORC binding to DNA. Finally, a structure of ORC was determined with endogenous DNA bound in the core revealing important differences between human and yeast origin recognition.
- W. M. Keck Structural Biology Laboratory, New York, United States.
Organizational Affiliation: