Structure of the cytoplasmic ring of the Xenopus laevis nuclear pore complex.
Zhu, X., Huang, G., Zeng, C., Zhan, X., Liang, K., Xu, Q., Zhao, Y., Wang, P., Wang, Q., Zhou, Q., Tao, Q., Liu, M., Lei, J., Yan, C., Shi, Y.(2022) Science 376: eabl8280-eabl8280
- PubMed: 35679404
- DOI: https://doi.org/10.1126/science.abl8280
- Primary Citation of Related Structures:
7FIK, 7FIL - PubMed Abstract:
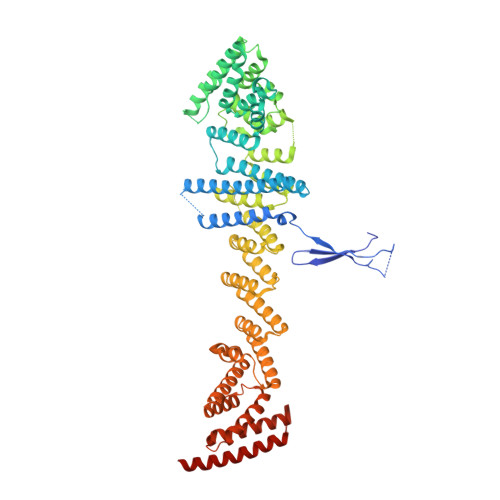
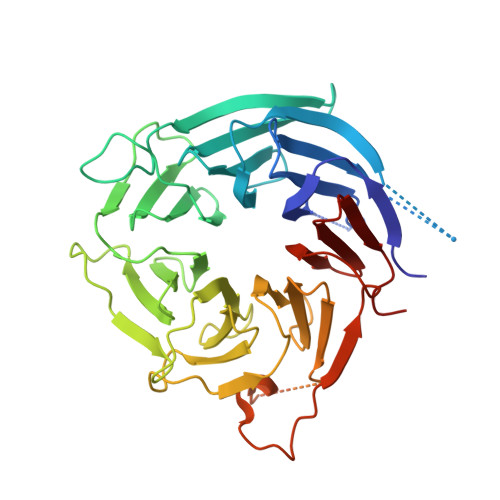
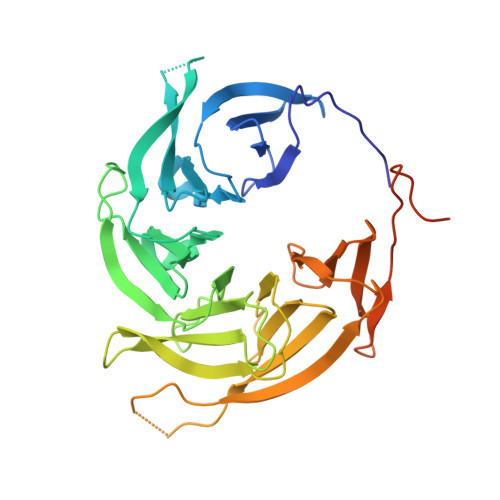
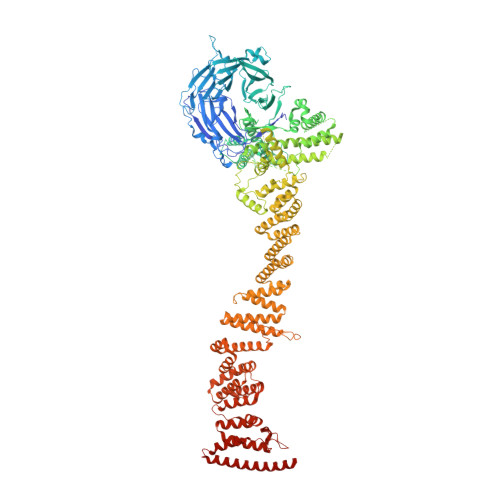
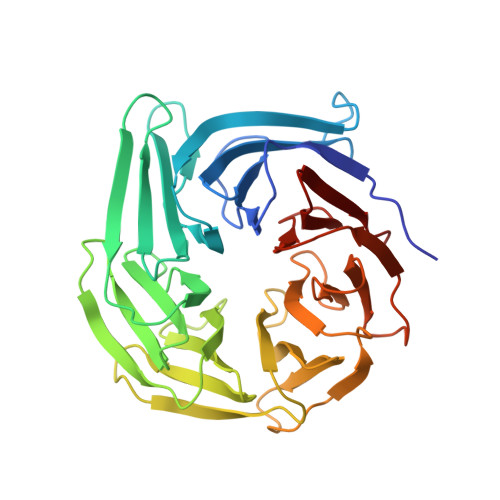
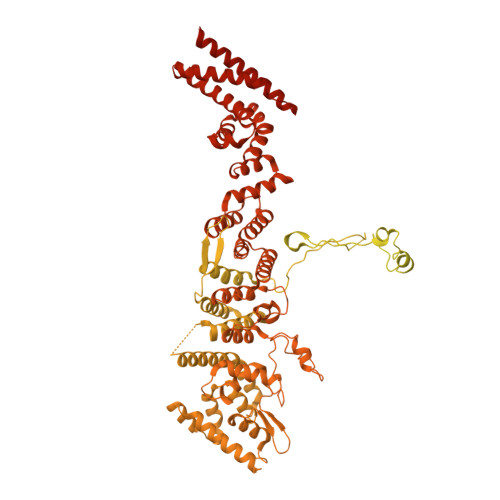
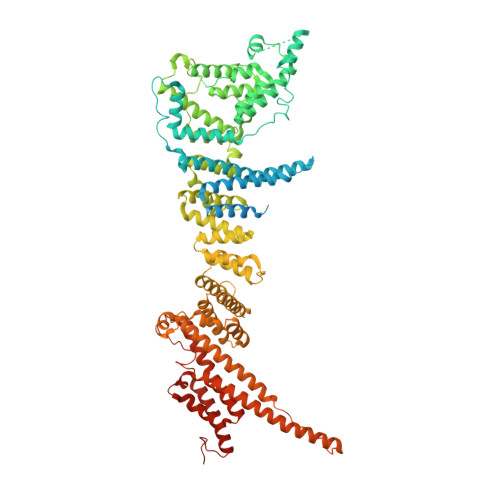
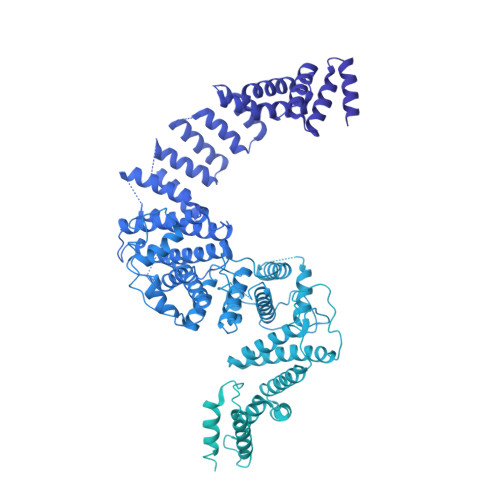
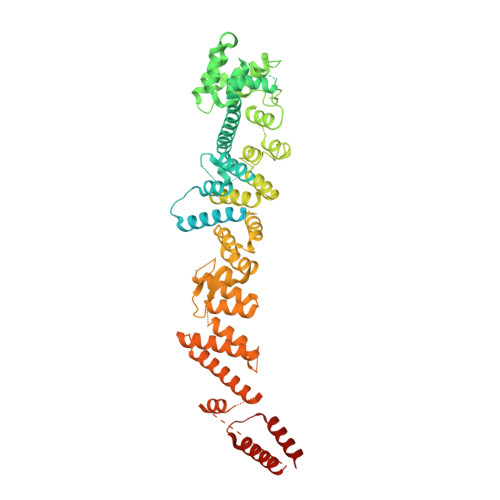
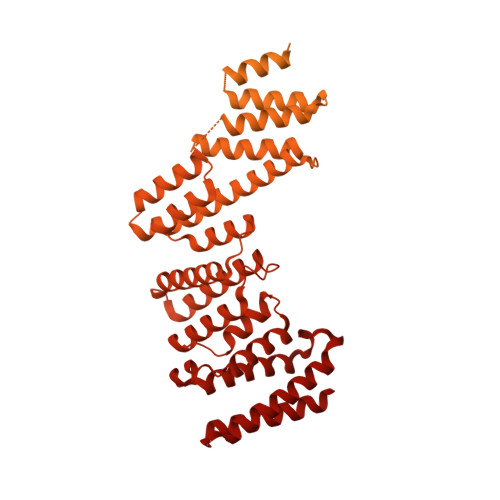
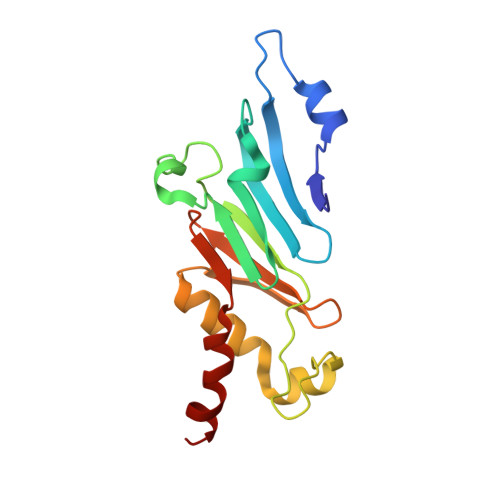
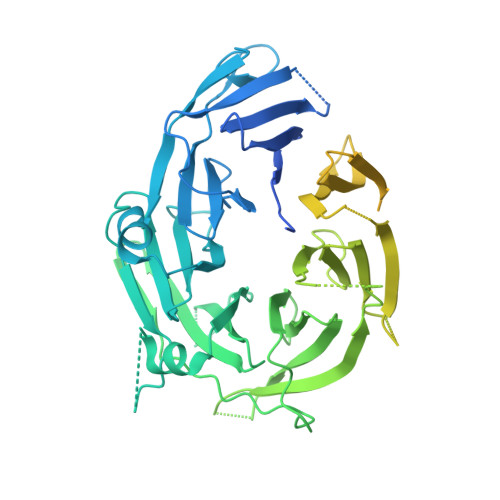
INTRODUCTION The nuclear pore complex (NPC) resides on the nuclear envelope (NE) and mediates nucleocytoplasmic cargo transport. As one of the largest cellular machineries, a vertebrate NPC consists of cytoplasmic filaments, a cytoplasmic ring (CR), an inner ring, a nuclear ring, a nuclear basket, and a luminal ring. Each NPC has eight repeating subunits. Structure determination of NPC is a prerequisite for understanding its functional mechanism. In the past two decades, integrative modeling, which combines x-ray structures of individual nucleoporins and subcomplexes with cryo-electron tomography reconstructions, has played a crucial role in advancing our knowledge about the NPC. The CR has been a major focus of structural investigation. The CR subunit of human NPC was reconstructed by cryo-electron tomography through subtomogram averaging to an overall resolution of ~20 Å, with local resolution up to ~15 Å. Each CR subunit comprises two Y-shaped multicomponent complexes known as the inner and outer Y complexes. Eight inner and eight outer Y complexes assemble in a head-to-tail fashion to form the proximal and distal rings, respectively, constituting the CR scaffold. To achieve higher resolution of the CR, we used single-particle cryo-electron microscopy (cryo-EM) to image the intact NPC from the NE of Xenopus laevis oocytes. Reconstructions of the core region and the Nup358 region of the X. laevis CR subunit had been achieved at average resolutions of 5 to 8 Å, allowing identification of secondary structural elements. RATIONALE Packing interactions among the components of the CR subunit were poorly defined by all previous EM maps. Additional components of the CR subunit are strongly suggested by the EM maps of 5- to 8-Å resolution but remain to be identified. Addressing these issues requires improved resolution of the cryo-EM reconstruction. Therefore, we may need to enhance sample preparation, optimize image acquisition, and develop an effective data-processing strategy. RESULTS To reduce conformational heterogeneity of the sample, we spread the opened NE onto the grids with minimal force and used the chemical cross-linker glutaraldehyde to stabilize the NPC. To alleviate orientation bias of the NPC, we tilted sample grids and imaged the sample with higher electron dose at higher angles. We improved the image-processing protocol. With these efforts, the average resolutions for the core and the Nup358 regions have been improved to 3.7 and 4.7 Å, respectively. The highest local resolution of the core region reaches 3.3 Å. In addition, a cryo-EM structure of the N-terminal α-helical domain of Nup358 has been resolved at 3.0-Å resolution. These EM maps allow the identification of five copies of Nup358, two copies of Nup93, two copies of Nup205, and two copies of Y complexes in each CR subunit. Relying on the EM maps and facilitated by AlphaFold prediction, we have generated a final model for the CR of the X. laevis NPC. Our model of the CR subunit includes 19,037 amino acids in 30 nucleoporins. A previously unknown C-terminal fragment of Nup160 was found to constitute a key part of the vertex, in which the short arm, long arm, and stem of the Y complex meet. The Nup160 C-terminal fragment directly binds the β-propeller proteins Seh1 and Sec13. Two Nup205 molecules, which do not contact each other, bind the inner and outer Y complexes through distinct interfaces. Conformational elasticity of the two Nup205 molecules may underlie their versatility in binding to different nucleoporins in the proximal and distal CR rings. Two Nup93 molecules, each comprising an N-terminal extended helix and an ACE1 domain, bridge the Y complexes and Nup205. Nup93 and Nup205 together play a critical role in mediating the contacts between neighboring CR subunits. Five Nup358 molecules, each in the shape of a shrimp tail and named "the clamp," hold the stems of both Y complexes. The innate conformational elasticity allows each Nup358 clamp to adapt to a distinct local environment for optimal interactions with neighboring nucleoporins. In each CR subunit, the α-helical nucleoporins appear to provide the conformational elasticity; the 12 β-propellers may strengthen the scaffold. CONCLUSION Our EM map-based model of the X. laevis CR subunit substantially expands the molecular mass over the reported composite models of vertebrate CR subunit. In addition to the Y complexes, five Nup358, two Nup205, and two Nup93 molecules constitute the key components of the CR. The improved EM maps reveal insights into the interfaces among the nucleoporins of the CR. [Figure: see text].
- Westlake Laboratory of Life Sciences and Biomedicine, Westlake University, 310024 Hangzhou, China.
Organizational Affiliation: