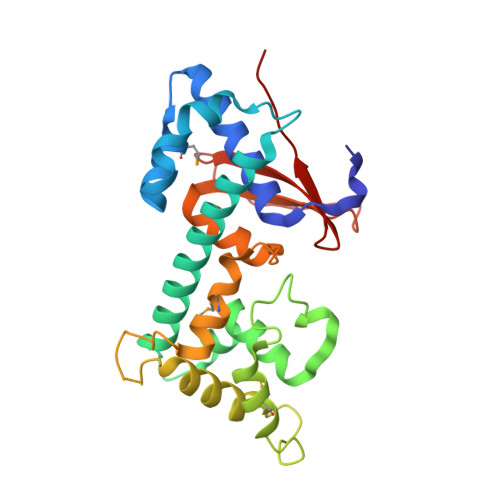
Structural Basis of Ubiquitin Recognition by a Bacterial Ovarian Tumor Deubiquitinase LotA.
Takekawa, N., Kubori, T., Iwai, T., Nagai, H., Imada, K.(2022) J Bacteriol 204: e0037621-e0037621
- PubMed: 34633867
- DOI: https://doi.org/10.1128/JB.00376-21
- Primary Citation of Related Structures:
7F9X - PubMed Abstract:
Pathogenic bacteria have acquired a vast array of eukaryotic-protein-like proteins via intimate interaction with host cells. Bacterial effector proteins that function as ubiquitin ligases and deubiquitinases (DUBs) are remarkable examples of such molecular mimicry. LotA, a Legionella pneumophila effector, belongs to the ovarian tumor (OTU) superfamily, which regulates diverse ubiquitin signals by their DUB activities. LotA harbors two OTU domains that have distinct reactivities; the first one is responsible for the cleavage of the K6-linked ubiquitin chain, and the second one shows an uncommon preference for long chains of ubiquitin. Here, we report the crystal structure of a middle domain of LotA (LotA M ), which contains the second OTU domain. LotA M consists of two distinct subdomains, a catalytic domain having high structural similarity with human OTU DUBs and an extended helical lobe (EHL) domain, which is characteristically conserved only in Legionella OTU DUBs. The docking simulation of LotA M with ubiquitin suggested that hydrophobic and electrostatic interactions between the EHL of LotA M and the C-terminal region of ubiquitin are crucial for the binding of ubiquitin to LotA M . The structure-based mutagenesis demonstrated that the acidic residue in the characteristic short helical segment termed the "helical arm" is essential for the enzymatic activity of LotA M . The EHL domain of the three Legionella OTU DUBs, LotA, LotB, and LotC, share the "helical arm" structure, suggesting that the EHL domain defines the Lot-OTUs as a unique class of DUBs. IMPORTANCE To successfully colonize, some pathogenic bacteria hijack the host ubiquitin system. Legionella OTU-like-DUBs (Lot-DUBs) are novel bacterial deubiquitinases found in effector proteins of L. pneumophila. LotA is a member of Lot-DUBs and has two OTU domains (OTU1 and OTU2). We determined the structure of a middle fragment of LotA (LotA M ), which includes OTU2. LotA M consists of the conserved catalytic domain and the Legionella OTUs-specific EHL domain. The docking simulation with ubiquitin and the mutational analysis suggested that the acidic surface in the EHL is essential for enzymatic activity. The structure of the EHL differs from those of other Lot-DUBs, suggesting that the variation of the EHL is related to the variable cleaving specificity of each DUB.
- Department of Macromolecular Science, Graduate School of Science, Osaka Universitygrid.136593.b, Osaka, Japan.
Organizational Affiliation: