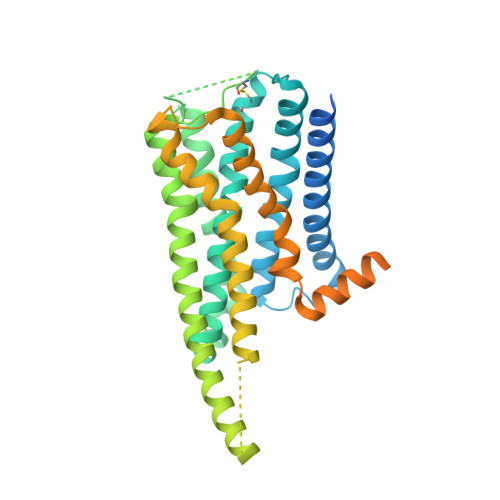
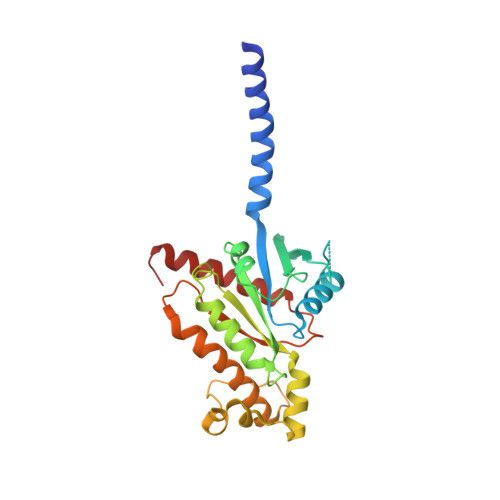
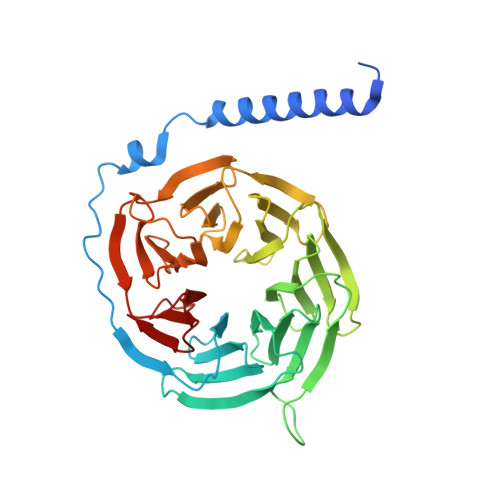
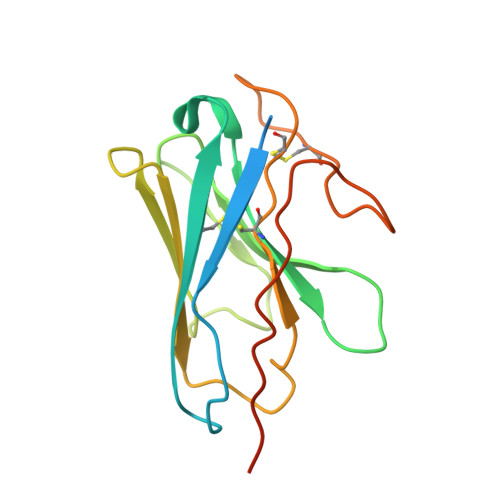
Structural insights into G protein activation by D1 dopamine receptor.
Teng, X., Chen, S., Wang, Q., Chen, Z., Wang, X., Huang, N., Zheng, S.(2022) Sci Adv 8: eabo4158-eabo4158
- PubMed: 35687690
- DOI: https://doi.org/10.1126/sciadv.abo4158
- Primary Citation of Related Structures:
7F0T, 7F1O, 7F1Z, 7F23, 7F24 - PubMed Abstract:
G protein-coupled receptors (GPCRs) comprise the largest family of membrane receptors and are the most important drug targets. An agonist-bound GPCR engages heterotrimeric G proteins and triggers the exchange of guanosine diphosphate (GDP) with guanosine triphosphate (GTP) to promote G protein activation. A complete understanding of molecular mechanisms of G protein activation has been hindered by a lack of structural information of GPCR-G protein complex in nucleotide-bound states. Here, we report the cryo-EM structures of the D1 dopamine receptor and mini-G s complex in the nucleotide-free and nucleotide-bound states. These structures reveal major conformational changes in Gα such as structural rearrangements of the carboxyl- and amino-terminal α helices that account for the release of GDP and the GTP-dependent dissociation of Gα from Gβγ subunits. As validated by biochemical and cellular signaling studies, our structures shed light into the molecular basis of the entire signaling events of GPCR-mediated G protein activation.
- Tsinghua Institute of Multidisciplinary Biomedical Research, Tsinghua University, Beijing, China.
Organizational Affiliation: