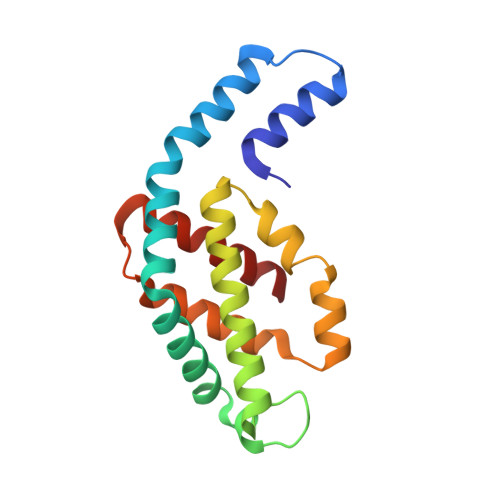
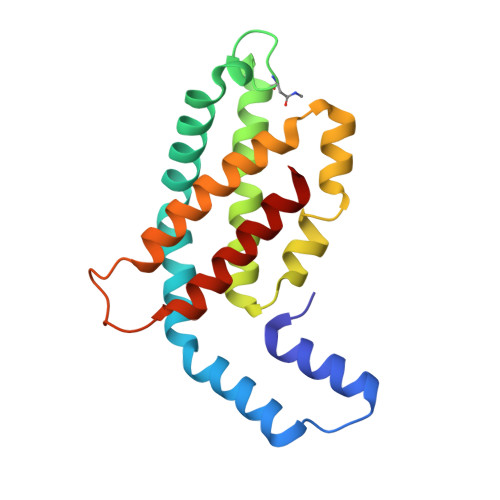
Non-conventional octameric structure of C-phycocyanin.
Minato, T., Teramoto, T., Adachi, N., Hung, N.K., Yamada, K., Kawasaki, M., Akutsu, M., Moriya, T., Senda, T., Ogo, S., Kakuta, Y., Yoon, K.S.(2021) Commun Biol 4: 1238-1238
- PubMed: 34716405
- DOI: https://doi.org/10.1038/s42003-021-02767-x
- Primary Citation of Related Structures:
7EFV, 7EFW, 7EH7, 7EH8 - PubMed Abstract:
C-phycocyanin (CPC), a blue pigment protein, is an indispensable component of giant phycobilisomes, which are light-harvesting antenna complexes in cyanobacteria that transfer energy efficiently to photosystems I and II. X-ray crystallographic and electron microscopy (EM) analyses have revealed the structure of CPC to be a closed toroidal hexamer by assembling two trimers. In this study, the structural characterization of non-conventional octameric CPC is reported for the first time. Analyses of the crystal and cryogenic EM structures of the native CPC from filamentous thermophilic cyanobacterium Thermoleptolyngbya sp. O-77 unexpectedly illustrated the coexistence of conventional hexamer and novel octamer. In addition, an unusual dimeric state, observed via analytical ultracentrifugation, was postulated to be a key intermediate structure in the assemble of the previously unobserved octamer. These observations provide new insights into the assembly processes of CPCs and the mechanism of energy transfer in the light-harvesting complexes.
- Department of Chemistry and Biochemistry, Graduate School of Engineering, Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka, 819-0395, Japan.
Organizational Affiliation: