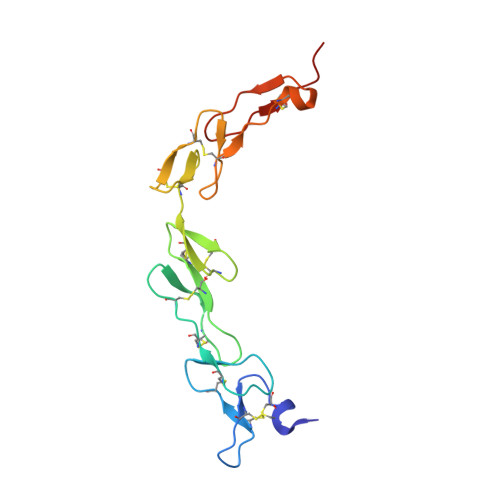
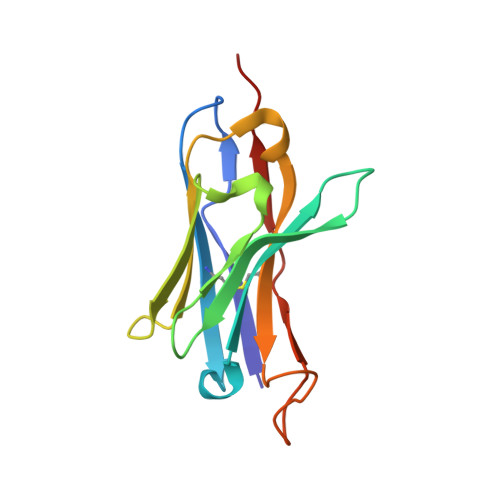
Generation of a safe and efficacious llama single-domain antibody fragment (vHH) targeting the membrane-proximal region of 4-1BB for engineering therapeutic bispecific antibodies for cancer.
Zhai, T., Wang, C., Xu, Y., Huang, W., Yuan, Z., Wang, T., Dai, S., Peng, S., Pang, T., Jiang, W., Huang, Y., Zou, Y., Xu, Y., Sun, J., Gong, X., Zhang, J., Tsun, A., Li, B., Miao, X.(2021) J Immunother Cancer 9
- PubMed: 34172514
- DOI: https://doi.org/10.1136/jitc-2020-002131
- Primary Citation of Related Structures:
7CZD, 7D4B - PubMed Abstract:
The discovery of checkpoint inhibitors towards cytotoxic T-lymphocyte protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) has been revolutionary for the treatment of cancers. These therapies have only offered an average of 20%-30% response rates across the tumor spectrum and the combination of agonists towards the tumor-necrosis superfamily members, such as 4-1BB and CD40, has shown potent efficacy in preclinical studies; however, these agonists have exhibited high degrees of toxicity with limited efficacy in human trials. In this study, we have generated a single-domain antibody towards a unique epitope of 4-1BB that limits its potential on-target toxicity while maintaining sufficient potency. This 4-1BB binder is ideal for use in the engineering of multispecific antibodies to localize 4-1BB activation within the tumor microenvironment, as shown here by a anti-PD-L1/4-1BB bispecific candidate (PM1003). To determine the functional activity of the 4-1BB- and PD-L1-binding elements of PM1003, in vitro luciferase reporter and primary cell assays were used to test the potency of programmed cell death 1 ligand 1 (PD-L1) blockade and PD-L1-mediated 4-1BB activation via cross-bridging. X-ray crystallography was conducted to resolve the binding epitopes of the respective binding arms, and accurate binding kinetics were determined using standard affinity measurement techniques. Human 4-1BB and/or PD-L1 knock-in mice were used in cancer models for testing the in vivo antitumor efficacy of PM1003, and safety was evaluated further. PM1003 shows potent activation of 4-1BB and blockade of PD-L1 in cell-based assays. 4-1BB activation was exerted through the bridging of PD-L1 on target cells and 4-1BB on effector cells. No PD-L1-independent activation of 4-1BB was observed. Through X-ray crystallography, a unique binding epitope in the cysteine-rich domain 4 (CRD4) region was resolved that provides high potency and potentially low on-target toxicity as determined by primary immune cell assays and toxicity evaluation in vivo . A unique single-domain antibody was discovered that binds to the CRD4 domain of 4-1BB. When incorporated into a 4-1BB/PD-L1 bispecific (PM1003), we have shown the potent inhibition of PD-L1 activity with 4-1BB agonism upon cross-bridging with PD-L1 in vitro . Antitumor activity with minimal toxicity was found in vivo . Thus, PM1003 is a uniquely differentiating and next generation therapeutic agent for cancer therapy.
- Shanghai Institute of Immunology, Department of Immunology and Microbiology, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Organizational Affiliation: