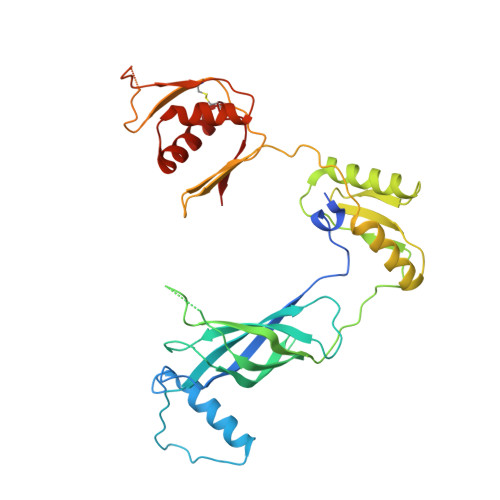
Structure of the molecular bushing of the bacterial flagellar motor.
Yamaguchi, T., Makino, F., Miyata, T., Minamino, T., Kato, T., Namba, K.(2021) Nat Commun 12: 4469-4469
- PubMed: 34294704
- DOI: https://doi.org/10.1038/s41467-021-24715-3
- Primary Citation of Related Structures:
7CLR - PubMed Abstract:
The basal body of the bacterial flagellum is a rotary motor that consists of several rings (C, MS and LP) and a rod. The LP ring acts as a bushing supporting the distal rod for its rapid and stable rotation without much friction. Here, we use electron cryomicroscopy to describe the LP ring structure around the rod, at 3.5 Å resolution, from Salmonella Typhimurium. The structure shows 26-fold rotational symmetry and intricate intersubunit interactions of each subunit with up to six partners, which explains the structural stability. The inner surface is charged both positively and negatively. Positive charges on the P ring (the part of the LP ring that is embedded within the peptidoglycan layer) presumably play important roles in its initial assembly around the rod with a negatively charged surface.
- Graduate School of Frontier Biosciences, Osaka University, Suita, Osaka, Japan.
Organizational Affiliation: