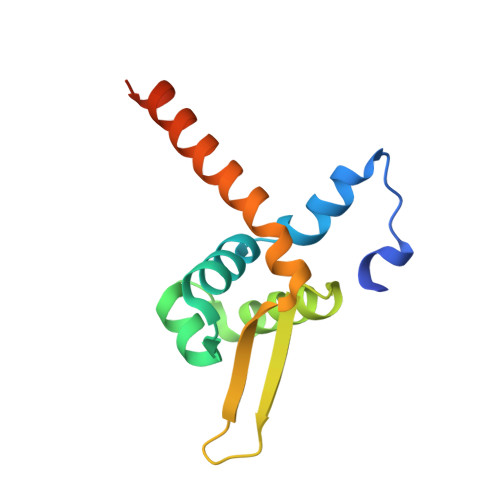
Genetically encoded formaldehyde sensors inspired by a protein intra-helical crosslinking reaction.
Zhu, R., Zhang, G., Jing, M., Han, Y., Li, J., Zhao, J., Li, Y., Chen, P.R.(2021) Nat Commun 12: 581-581
- PubMed: 33495458
- DOI: https://doi.org/10.1038/s41467-020-20754-4
- Primary Citation of Related Structures:
7BZD, 7BZE, 7BZG - PubMed Abstract:
Formaldehyde (FA) has long been considered as a toxin and carcinogen due to its damaging effects to biological macromolecules, but its beneficial roles have been increasingly appreciated lately. Real-time monitoring of this reactive molecule in living systems is highly desired in order to decipher its physiological and/or pathological functions, but a genetically encoded FA sensor is currently lacking. We herein adopt a structure-based study of the underlying mechanism of the FA-responsive transcription factor HxlR from Bacillus subtilis, which shows that HxlR recognizes FA through an intra-helical cysteine-lysine crosslinking reaction at its N-terminal helix α1, leading to conformational change and transcriptional activation. By leveraging this FA-induced intra-helical crosslinking and gain-of-function reorganization, we develop the genetically encoded, reaction-based FA sensor-FAsor, allowing spatial-temporal visualization of FA in mammalian cells and mouse brain tissues.
- Synthetic and Functional Biomolecules Center, Beijing National Laboratory for Molecular Sciences, College of Chemistry and Molecular Engineering, Peking University, 100871, Beijing, China.
Organizational Affiliation: