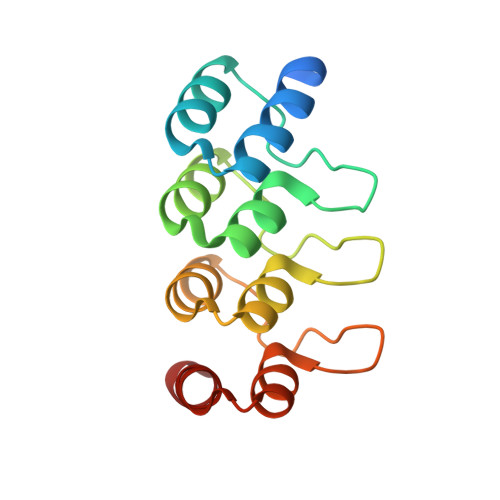
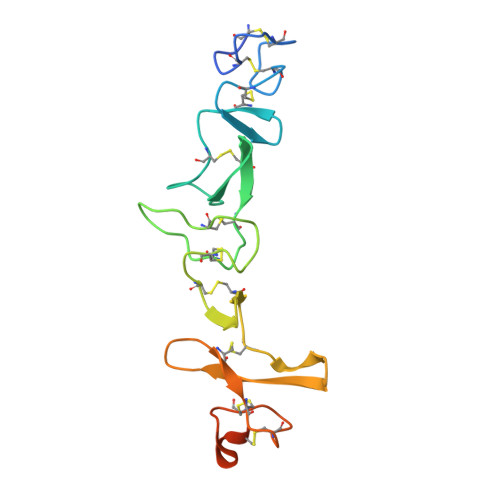
Crystal structures of HER3 extracellular domain 4 in complex with the designed ankyrin-repeat protein D5.
Radom, F., Vonrhein, C., Mittl, P.R.E., Pluckthun, A.(2021) Acta Crystallogr F Struct Biol Commun 77: 192-201
- PubMed: 34196609
- DOI: https://doi.org/10.1107/S2053230X21006002
- Primary Citation of Related Structures:
7BHE, 7BHF - PubMed Abstract:
The members of the human epidermal growth factor receptor (HER) family are among the most intensely studied oncological targets. HER3 (ErbB3), which had long been neglected, has emerged as a key oncogene, regulating the activity of other receptors and being involved in progression and tumor escape in multiple types of cancer. Designed ankyrin-repeat proteins (DARPins) serve as antibody mimetics that have proven to be useful in the clinic, in diagnostics and in research. DARPins have previously been selected against EGFR (HER1), HER2 and HER4. In particular, their combination into bivalent binders that separate or lock receptors in their inactive conformation has proved to be a promising strategy for the design of potent anticancer therapeutics. Here, the selection of DARPins targeting extracellular domain 4 of HER3 (HER3d4) is described. One of the selected DARPins, D5, in complex with HER3d4 crystallized in two closely related crystal forms that diffracted to 2.3 and 2.0 Å resolution, respectively. The DARPin D5 epitope comprises HER3d4 residues 568-577. These residues also contribute to interactions within the tethered (inactive) and extended (active) conformations of the extracellular domain of HER3.
- Department of Biochemistry, University of Zurich, Winterthurerstrasse 190, CH-8057 Zürich, Switzerland.
Organizational Affiliation: