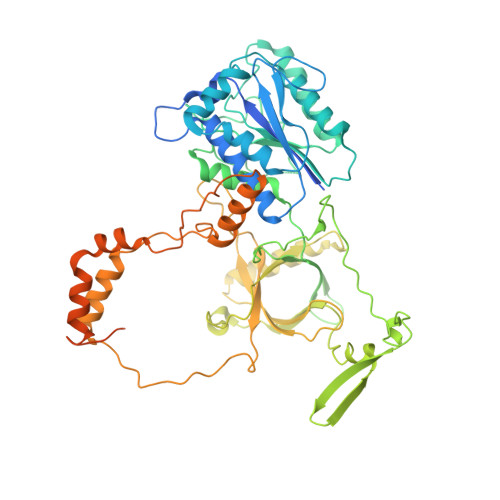
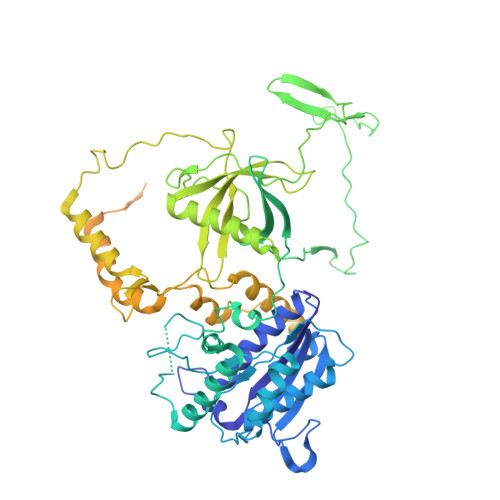
SAP domain forms a flexible part of DNA aperture in Ku70/80.
Hnizda, A., Tesina, P., Nguyen, T.B., Kukacka, Z., Kater, L., Chaplin, A.K., Beckmann, R., Ascher, D.B., Novak, P., Blundell, T.L.(2021) FEBS J 288: 4382-4393
- PubMed: 33511782
- DOI: https://doi.org/10.1111/febs.15732
- Primary Citation of Related Structures:
7AXZ - PubMed Abstract:
Nonhomologous end joining (NHEJ) is a DNA repair mechanism that religates double-strand DNA breaks to maintain genomic integrity during the entire cell cycle. The Ku70/80 complex recognizes DNA breaks and serves as an essential hub for recruitment of NHEJ components. Here, we describe intramolecular interactions of the Ku70 C-terminal domain, known as the SAP domain. Using single-particle cryo-electron microscopy, mass spectrometric analysis of intermolecular cross-linking and molecular modelling simulations, we captured variable positions of the SAP domain depending on DNA binding. The first position was localized at the DNA aperture in the Ku70/80 apo form but was not observed in the DNA-bound state. The second position, which was observed in both apo and DNA-bound states, was found below the DNA aperture, close to the helical arm of Ku70. The localization of the SAP domain in the DNA aperture suggests a function as a flexible entry gate for broken DNA. DATABASES: EM maps have been deposited in EMDB (EMD-11933). Coordinates have been deposited in Protein Data Bank (PDB 7AXZ). Other data are available from corresponding authors upon a request.
- Department of Biochemistry, University of Cambridge, Cambridge, UK.
Organizational Affiliation: