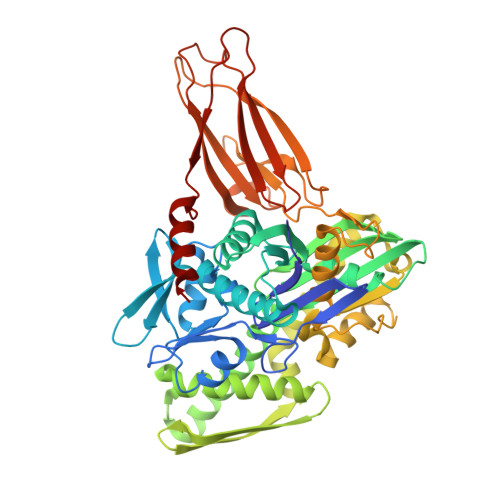
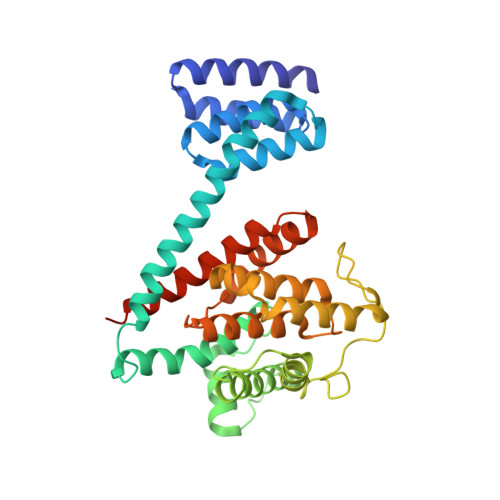
Specificity of AMPylation of the human chaperone BiP is mediated by TPR motifs of FICD.
Fauser, J., Gulen, B., Pogenberg, V., Pett, C., Pourjafar-Dehkordi, D., Krisp, C., Hopfner, D., Konig, G., Schluter, H., Feige, M.J., Zacharias, M., Hedberg, C., Itzen, A.(2021) Nat Commun 12: 2426-2426
- PubMed: 33893288
- DOI: https://doi.org/10.1038/s41467-021-22596-0
- Primary Citation of Related Structures:
6ZMD - PubMed Abstract:
To adapt to fluctuating protein folding loads in the endoplasmic reticulum (ER), the Hsp70 chaperone BiP is reversibly modified with adenosine monophosphate (AMP) by the ER-resident Fic-enzyme FICD/HYPE. The structural basis for BiP binding and AMPylation by FICD has remained elusive due to the transient nature of the enzyme-substrate-complex. Here, we use thiol-reactive derivatives of the cosubstrate adenosine triphosphate (ATP) to covalently stabilize the transient FICD:BiP complex and determine its crystal structure. The complex reveals that the TPR-motifs of FICD bind specifically to the conserved hydrophobic linker of BiP and thus mediate specificity for the domain-docked conformation of BiP. Furthermore, we show that both AMPylation and deAMPylation of BiP are not directly regulated by the presence of unfolded proteins. Together, combining chemical biology, crystallography and biochemistry, our study provides structural insights into a key regulatory mechanism that safeguards ER homeostasis.
- Department of Biochemistry and Signal Transduction, University Medical Center Hamburg-Eppendorf (UKE), Hamburg, Germany.
Organizational Affiliation: