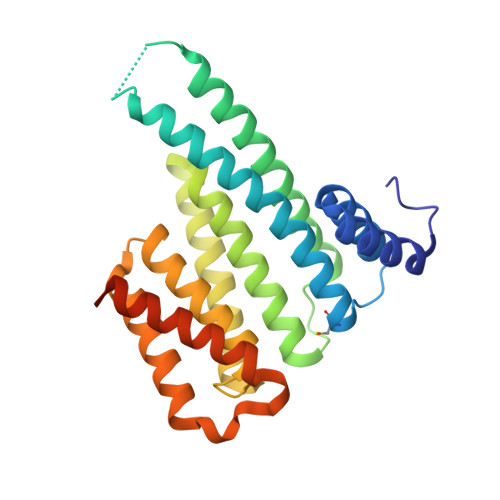
Supramolecular Enhancement of a Natural 14-3-3 Protein Ligand.
Guillory, X., Hadrovic, I., de Vink, P.J., Sowislok, A., Brunsveld, L., Schrader, T., Ottmann, C.(2021) J Am Chem Soc 143: 13495-13500
- PubMed: 34427424
- DOI: https://doi.org/10.1021/jacs.1c07095
- Primary Citation of Related Structures:
6Y7T - PubMed Abstract:
Rational design of protein-protein interaction (PPI) inhibitors is challenging. Connecting a general supramolecular protein binder with a specific peptidic ligand provides a novel conceptual approach. Thus, lysine-specific molecular tweezers were conjugated to a peptide-based 14-3-3 ligand and produced a strong PPI inhibitor with 100-fold elevated protein affinity. X-ray crystal structure elucidation of this supramolecular directed assembly provides unique molecular insight into the binding mode and fully aligns with Molecular Dynamics (MD) simulations. This new supramolecular chemical biology concept opens the path to novel chemical tools for studying PPIs.
- Laboratory of Chemical Biology, Department of Biomedical Engineering and Institute for Complex Molecular System, Eindhoven University of Technology, (TU/e) Den Dolech 2, 5612 AZ Eindhoven, The Netherlands.
Organizational Affiliation: