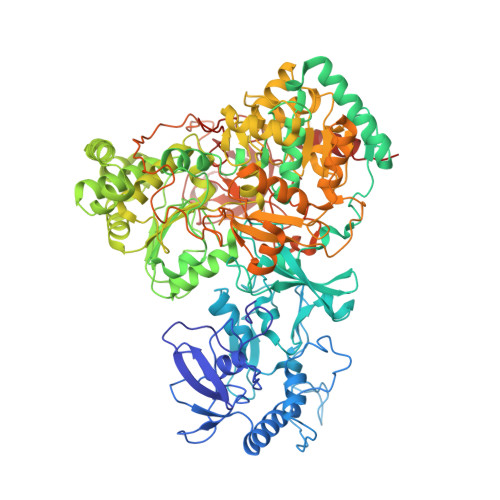
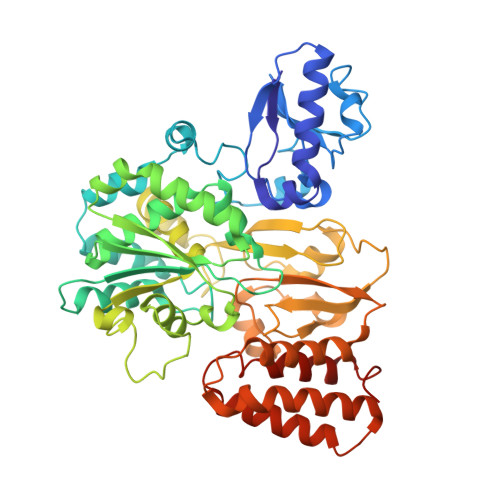
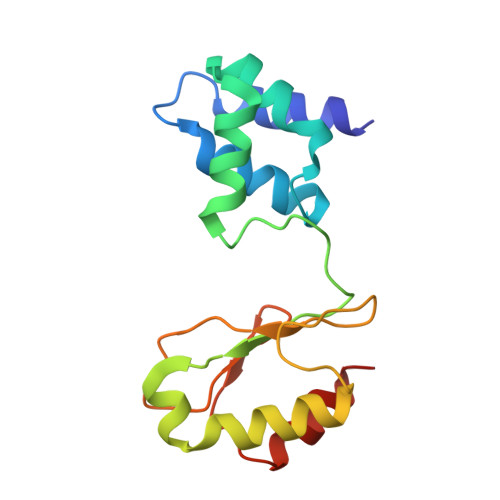
Cryo-EM structures reveal intricate Fe-S cluster arrangement and charging in Rhodobacter capsulatus formate dehydrogenase.
Radon, C., Mittelstadt, G., Duffus, B.R., Burger, J., Hartmann, T., Mielke, T., Teutloff, C., Leimkuhler, S., Wendler, P.(2020) Nat Commun 11: 1912-1912
- PubMed: 32313256
- DOI: https://doi.org/10.1038/s41467-020-15614-0
- Primary Citation of Related Structures:
6TG9, 6TGA - PubMed Abstract:
Metal-containing formate dehydrogenases (FDH) catalyse the reversible oxidation of formate to carbon dioxide at their molybdenum or tungsten active site. They display a diverse subunit and cofactor composition, but structural information on these enzymes is limited. Here we report the cryo-electron microscopic structures of the soluble Rhodobacter capsulatus FDH (RcFDH) as isolated and in the presence of reduced nicotinamide adenine dinucleotide (NADH). RcFDH assembles into a 360 kDa dimer of heterotetramers revealing a putative interconnection of electron pathway chains. In the presence of NADH, the RcFDH structure shows charging of cofactors, indicative of an increased electron load.
- Institute of Biochemistry and Biology, Department of Biochemistry, University of Potsdam, Karl-Liebknecht Strasse 24-25, 14476, Potsdam-Golm, Germany.
Organizational Affiliation: