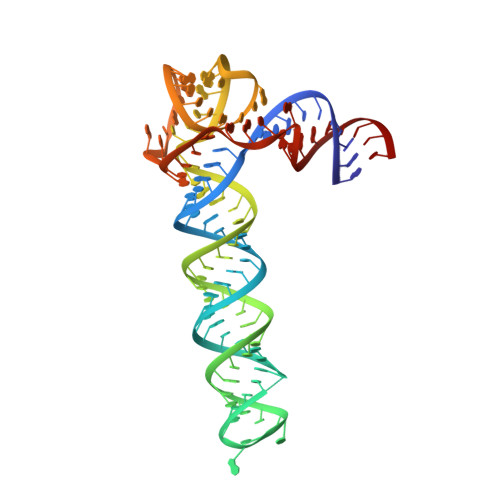
Crystal structure of an adenovirus virus-associated RNA.
Hood, I.V., Gordon, J.M., Bou-Nader, C., Henderson, F.E., Bahmanjah, S., Zhang, J.(2019) Nat Commun 10: 2871-2871
- PubMed: 31253805
- DOI: https://doi.org/10.1038/s41467-019-10752-6
- Primary Citation of Related Structures:
6OL3 - PubMed Abstract:
Adenovirus Virus-Associated (VA) RNAs are the first discovered viral noncoding RNAs. By mimicking double-stranded RNAs (dsRNAs), the exceptionally abundant, multifunctional VA RNAs sabotage host machineries that sense, transport, process, or edit dsRNAs. How VA-I suppresses PKR activation despite its strong dsRNA character, and inhibits the crucial antiviral kinase to promote viral translation, remains largely unknown. Here, we report a 2.7 Å crystal structure of VA-I RNA. The acutely bent VA-I features an unusually structured apical loop, a wobble-enriched, coaxially stacked apical and tetra-stems necessary and sufficient for PKR inhibition, and a central domain pseudoknot that resembles codon-anticodon interactions and prevents PKR activation by VA-I. These global and local structural features collectively define VA-I as an archetypal PKR inhibitor made of RNA. The study provides molecular insights into how viruses circumnavigate cellular rules of self vs non-self RNAs to not only escape, but further compromise host innate immunity.
- Laboratory of Molecular Biology, National Institute of Diabetes and Digestive and Kidney Diseases, 50 South Drive, Room 4503, Bethesda, MD, 20892, USA.
Organizational Affiliation: