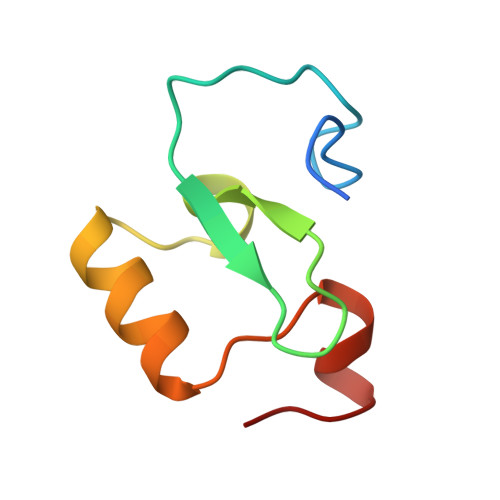
Selective binding of the PHD6 finger of MLL4 to histone H4K16ac links MLL4 and MOF.
Zhang, Y., Jang, Y., Lee, J.E., Ahn, J., Xu, L., Holden, M.R., Cornett, E.M., Krajewski, K., Klein, B.J., Wang, S.P., Dou, Y., Roeder, R.G., Strahl, B.D., Rothbart, S.B., Shi, X., Ge, K., Kutateladze, T.G.(2019) Nat Commun 10: 2314-2314
- PubMed: 31127101
- DOI: https://doi.org/10.1038/s41467-019-10324-8
- Primary Citation of Related Structures:
6O7G - PubMed Abstract:
Histone methyltransferase MLL4 is centrally involved in transcriptional regulation and is often mutated in human diseases, including cancer and developmental disorders. MLL4 contains a catalytic SET domain that mono-methylates histone H3K4 and seven PHD fingers of unclear function. Here, we identify the PHD6 finger of MLL4 (MLL4-PHD6) as a selective reader of the epigenetic modification H4K16ac. The solution NMR structure of MLL4-PHD6 in complex with a H4K16ac peptide along with binding and mutational analyses reveal unique mechanistic features underlying recognition of H4K16ac. Genomic studies show that one third of MLL4 chromatin binding sites overlap with H4K16ac-enriched regions in vivo and that MLL4 occupancy in a set of genomic targets depends on the acetyltransferase activity of MOF, a H4K16ac-specific acetyltransferase. The recognition of H4K16ac is conserved in the PHD7 finger of paralogous MLL3. Together, our findings reveal a previously uncharacterized acetyllysine reader and suggest that selective targeting of H4K16ac by MLL4 provides a direct functional link between MLL4, MOF and H4K16 acetylation.
- Department of Pharmacology, University of Colorado School of Medicine, Aurora, CO, 80045, USA.
Organizational Affiliation: