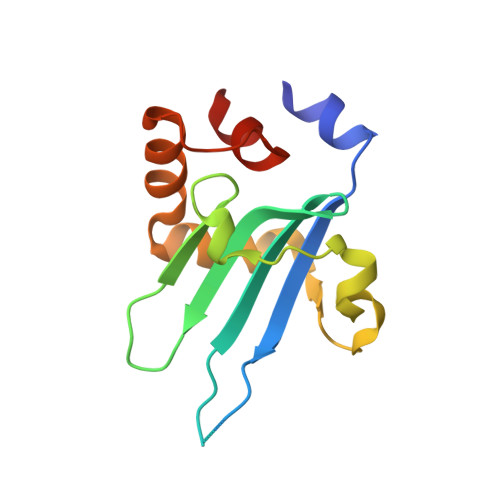
Structure of Myosin VI/Tom1 complex reveals a cargo recognition mode of Myosin VI for tethering.
Hu, S., Guo, Y., Wang, Y., Li, Y., Fu, T., Zhou, Z., Wang, Y., Liu, J., Pan, L.(2019) Nat Commun 10: 3459-3459
- PubMed: 31371777
- DOI: https://doi.org/10.1038/s41467-019-11481-6
- Primary Citation of Related Structures:
6J56 - PubMed Abstract:
Myosin VI plays crucial roles in diverse cellular processes. In autophagy, Myosin VI can facilitate the maturation of autophagosomes through interactions with Tom1 and the autophagy receptors, Optineurin, NDP52 and TAX1BP1. Here, we report the high-resolution crystal structure of the C-terminal cargo-binding domain (CBD) of Myosin VI in complex with Tom1, which elucidates the mechanistic basis underpinning the specific interaction between Myosin VI and Tom1, and uncovers that the C-terminal CBD of Myosin VI adopts a unique cargo recognition mode to interact with Tom1 for tethering. Furthermore, we show that Myosin VI can serve as a bridging adaptor to simultaneously interact with Tom1 and autophagy receptors through two distinct interfaces. In all, our findings provide mechanistic insights into the interactions of Myosin VI with Tom1 and relevant autophagy receptors, and are valuable for further understanding the functions of these proteins in autophagy and the cargo recognition modes of Myosin VI.
- State Key Laboratory of Bioorganic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 200032, Shanghai, China.
Organizational Affiliation: