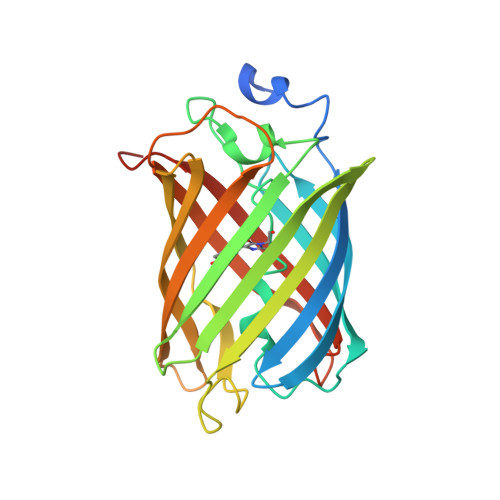
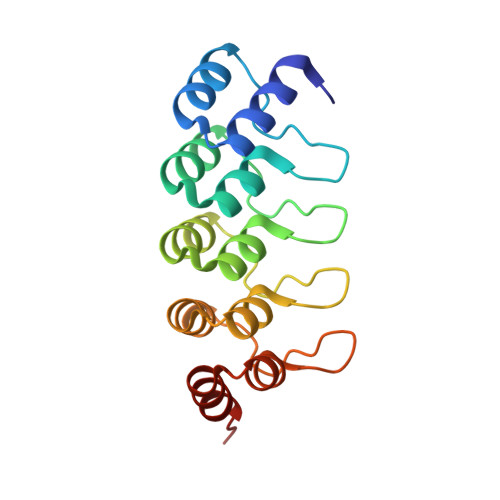
DARPins recognizing mTFP1 as novel reagents forin vitroandin vivoprotein manipulations.
Vigano, M.A., Bieli, D., Schaefer, J.V., Jakob, R.P., Matsuda, S., Maier, T., Pluckthun, A., Affolter, M.(2018) Biol Open 7
- PubMed: 30237292
- DOI: https://doi.org/10.1242/bio.036749
- Primary Citation of Related Structures:
6FP7, 6FP8, 6FP9, 6FPA, 6FPB - PubMed Abstract:
Over the last few years, protein-based affinity reagents have proven very helpful in cell and developmental biology. While many of these versatile small proteins can be expressed both in the intracellular and extracellular milieu in cultured cells and in living organisms, they can also be functionalized by fusing them to different protein domains in order to regulate or modulate their target proteins in diverse manners. For example, protein binders have been employed to degrade, trap, localize or enzymatically modify specific target proteins. Whereas binders to many endogenous proteins or small protein tags have been generated, several affinity reagents against fluorescent proteins have also been created and used to manipulate target proteins tagged with the corresponding fluorescent protein. Both of these approaches have resulted in improved methods for cell biological and developmental studies. While binders against GFP and mCherry have been previously isolated and validated, we now report the generation and utilization of designed ankyrin repeat proteins (DARPins) against the monomeric teal fluorescent protein 1 (mTFP1). Here we use the generated DARPins to delocalize Rab proteins to the nuclear compartment, in which they cannot fulfil their regular functions anymore. In the future, such manipulations might enable the production of acute loss-of-function phenotypes in different cell types or in living organisms based on direct protein manipulation rather than on genetic loss-of-function analyses.
- Growth and Development, Biozentrum, University of Basel, Klingelbergstrasse 70, CH-4056 Basel, Switzerland.
Organizational Affiliation: