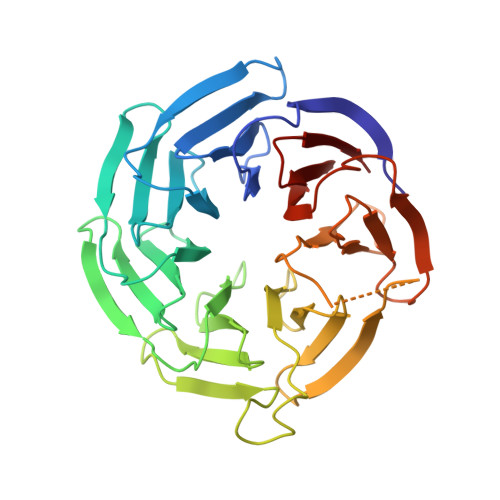
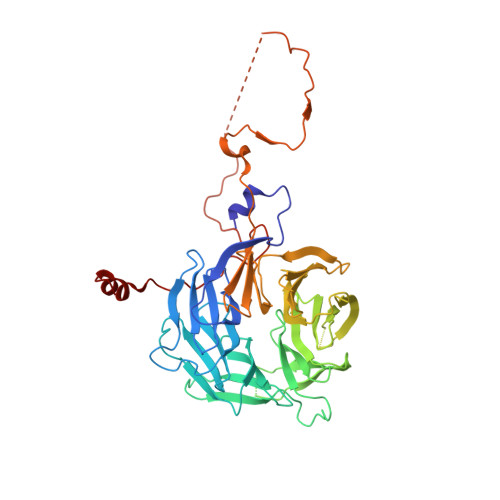
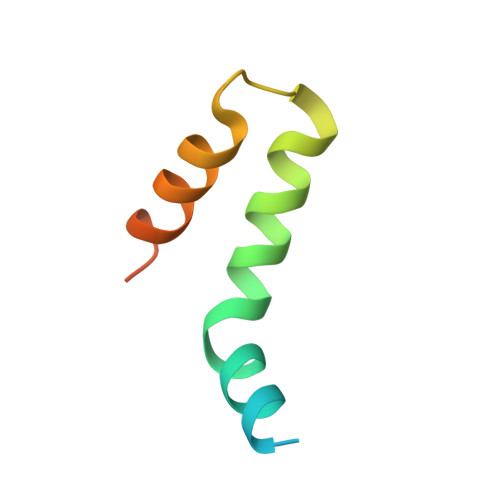
Crystal Structure of the COMPASS H3K4 Methyltransferase Catalytic Module.
Hsu, P.L., Li, H., Lau, H.T., Leonen, C., Dhall, A., Ong, S.E., Chatterjee, C., Zheng, N.(2018) Cell 174: 1106
- PubMed: 30100181
- DOI: https://doi.org/10.1016/j.cell.2018.06.038
- Primary Citation of Related Structures:
6CHG - PubMed Abstract:
The SET1/MLL family of histone methyltransferases is conserved in eukaryotes and regulates transcription by catalyzing histone H3K4 mono-, di-, and tri-methylation. These enzymes form a common five-subunit catalytic core whose assembly is critical for their basal and regulated enzymatic activities through unknown mechanisms. Here, we present the crystal structure of the intact yeast COMPASS histone methyltransferase catalytic module consisting of Swd1, Swd3, Bre2, Sdc1, and Set1. The complex is organized by Swd1, whose conserved C-terminal tail not only nucleates Swd3 and a Bre2-Sdc1 subcomplex, but also joins Set1 to construct a regulatory pocket next to the catalytic site. This inter-subunit pocket is targeted by a previously unrecognized enzyme-modulating motif in Swd3 and features a doorstop-style mechanism dictating substrate selectivity among SET1/MLL family members. By spatially mapping the functional components of COMPASS, our results provide a structural framework for understanding the multifaceted functions and regulation of the H3K4 methyltransferase family.
- Department of Pharmacology, University of Washington, Seattle, WA 98195, USA.
Organizational Affiliation: