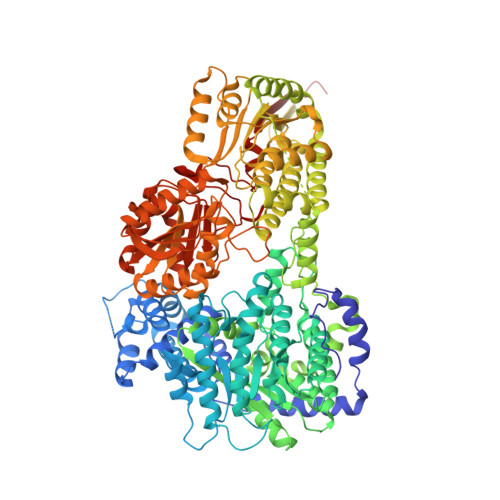
Structural Basis for the Substrate Inhibition of Proline Utilization A by Proline.
Korasick, D.A., Pemberton, T.A., Arentson, B.W., Becker, D.F., Tanner, J.J.(2017) Molecules 23
- PubMed: 29295473
- DOI: https://doi.org/10.3390/molecules23010032
- Primary Citation of Related Structures:
6BSN - PubMed Abstract:
Proline utilization A (PutA) is a bifunctional flavoenzyme that catalyzes the two-step oxidation of l-proline to l-glutamate using spatially separated proline dehydrogenase (PRODH) and l-glutamate-γ-semialdehyde dehydrogenase (GSALDH) active sites. Substrate inhibition of the coupled PRODH-GSALDH reaction by proline is a common kinetic feature of PutAs, yet the structural basis for this phenomenon remains unknown. To understand the mechanism of substrate inhibition, we determined the 2.15 Å resolution crystal structure of Bradyrhizobium japonicum PutA complexed with proline. Proline was discovered in five locations remote from the PRODH active site. Most notably, strong electron density indicated that proline bound tightly to the GSAL binding site of the GSALDH active site. The pose and interactions of proline bound in this site are remarkably similar to those of the natural aldehyde substrate, GSAL, implying that proline inhibits the GSALDH reaction of PutA. Kinetic measurements show that proline is a competitive inhibitor of the PutA GSALDH reaction. Together, the structural and kinetic data show that substrate inhibition of the PutA coupled reaction is due to proline binding in the GSAL site.
- Department of Biochemistry, University of Missouri, Columbia, MO 65211, USA. korasickd@missouri.edu.
Organizational Affiliation: