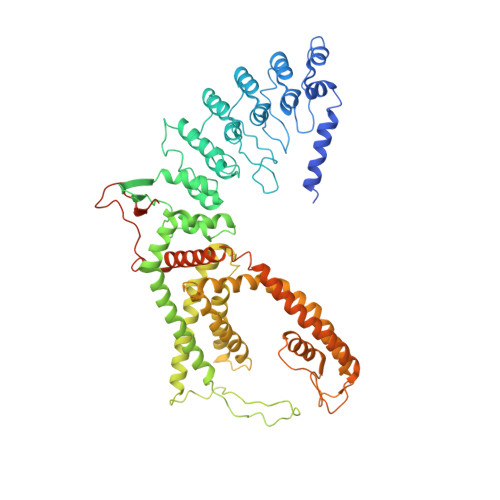
Opening of the human epithelial calcium channel TRPV6.
McGoldrick, L.L., Singh, A.K., Saotome, K., Yelshanskaya, M.V., Twomey, E.C., Grassucci, R.A., Sobolevsky, A.I.(2018) Nature 553: 233-237
- PubMed: 29258289
- DOI: https://doi.org/10.1038/nature25182
- Primary Citation of Related Structures:
6BO8, 6BO9, 6BOA, 6BOB - PubMed Abstract:
Calcium-selective transient receptor potential vanilloid subfamily member 6 (TRPV6) channels play a critical role in calcium uptake in epithelial tissues. Altered TRPV6 expression is associated with a variety of human diseases, including cancers. TRPV6 channels are constitutively active and their open probability depends on the lipidic composition of the membrane in which they reside; it increases substantially in the presence of phosphatidylinositol 4,5-bisphosphate. Crystal structures of detergent-solubilized rat TRPV6 in the closed state have previously been solved. Corroborating electrophysiological results, these structures demonstrated that the Ca 2+ selectivity of TRPV6 arises from a ring of aspartate side chains in the selectivity filter that binds Ca 2+ tightly. However, how TRPV6 channels open and close their pores for ion permeation has remained unclear. Here we present cryo-electron microscopy structures of human TRPV6 in the open and closed states. The channel selectivity filter adopts similar conformations in both states, consistent with its explicit role in ion permeation. The iris-like channel opening is accompanied by an α-to-π-helical transition in the pore-lining transmembrane helix S6 at an alanine hinge just below the selectivity filter. As a result of this transition, the S6 helices bend and rotate, exposing different residues to the ion channel pore in the open and closed states. This gating mechanism, which defines the constitutive activity of TRPV6, is, to our knowledge, unique among tetrameric ion channels and provides structural insights for understanding their diverse roles in physiology and disease.
- Department of Biochemistry and Molecular Biophysics, Columbia University, 650 West 168th Street, New York, New York 10032, USA.
Organizational Affiliation: