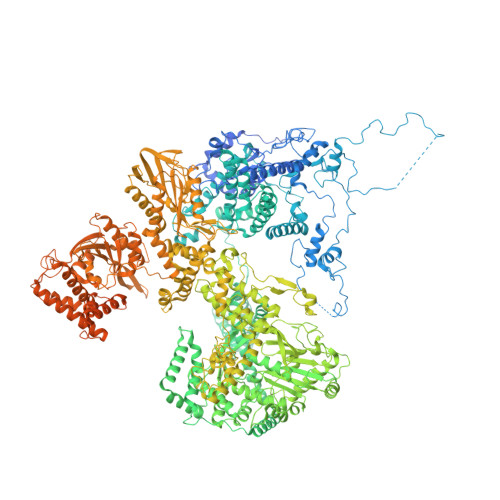
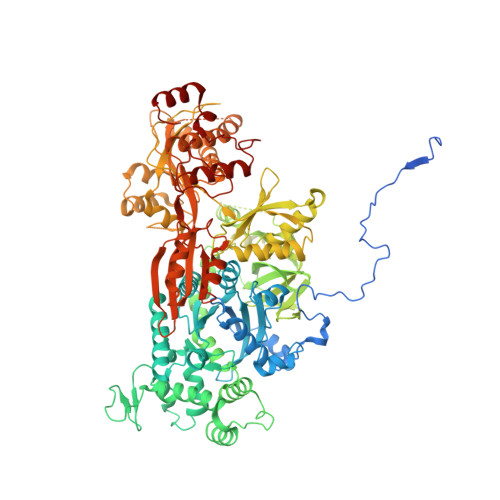
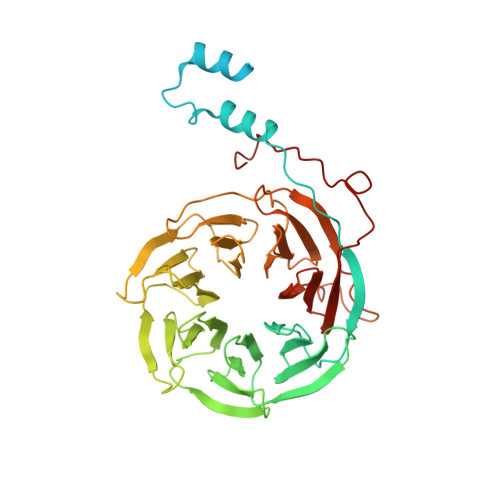
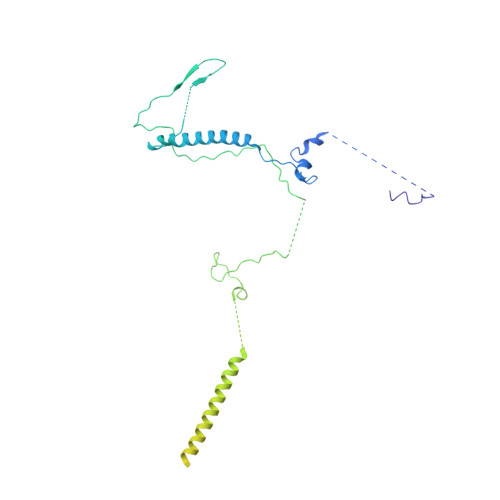
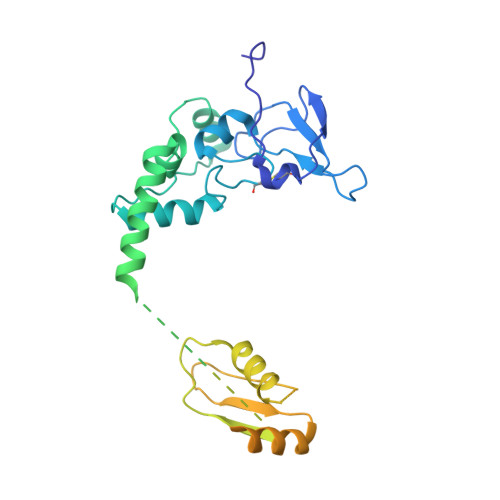
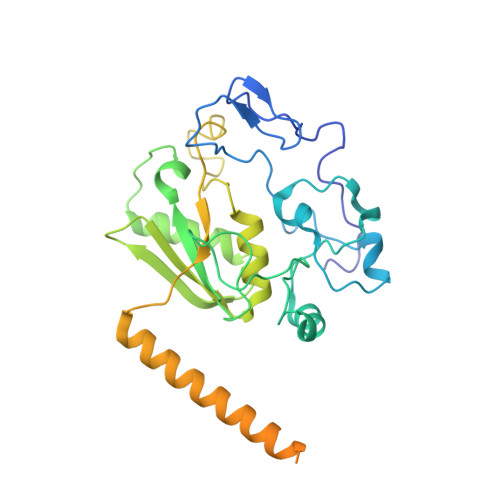
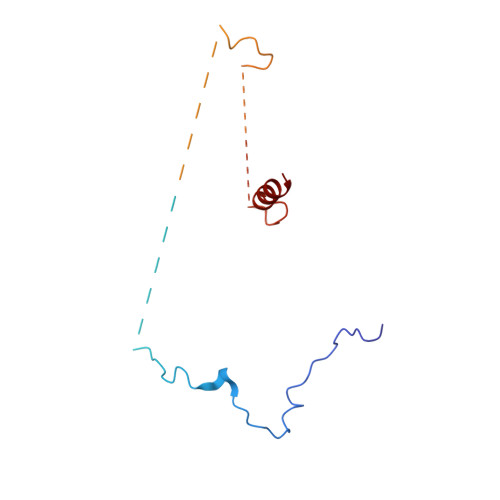
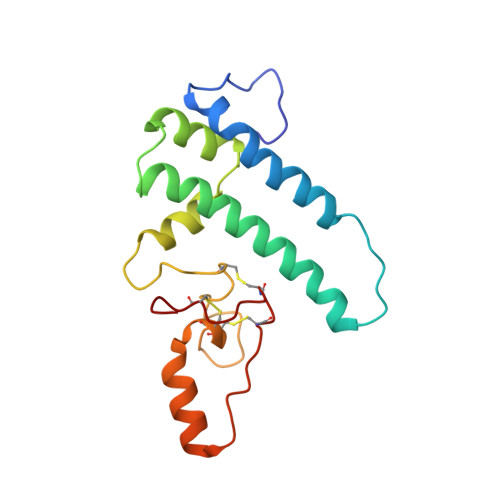
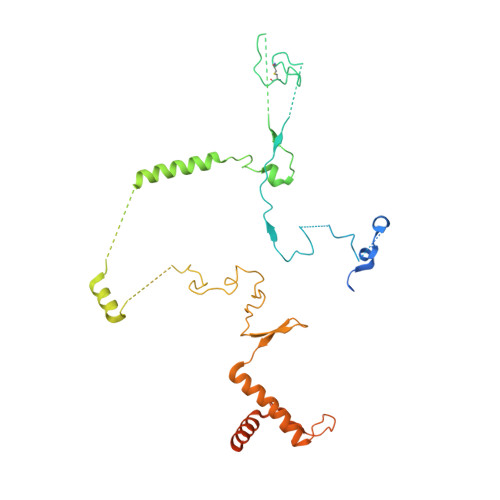
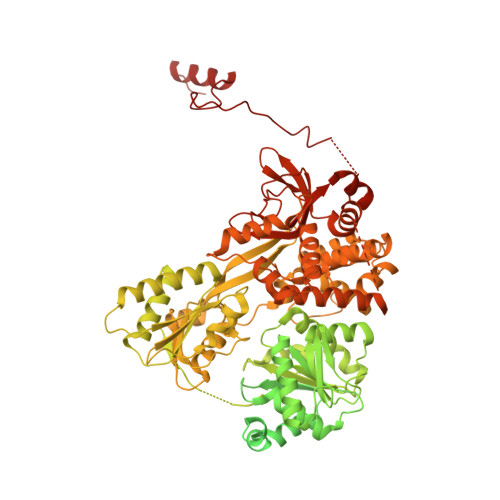
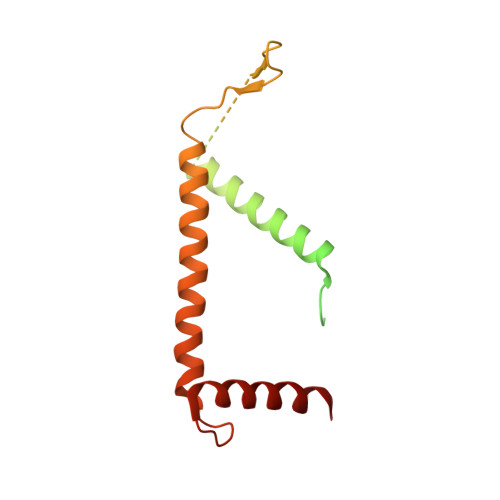
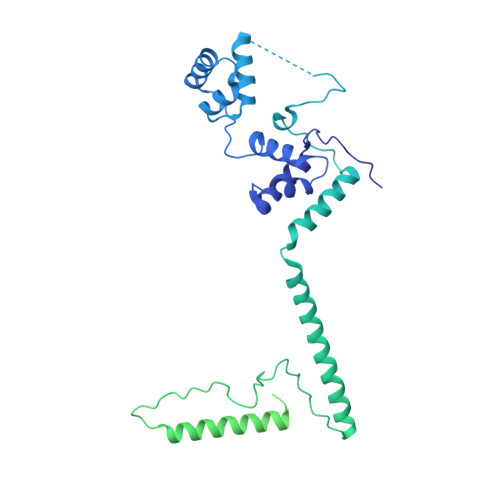
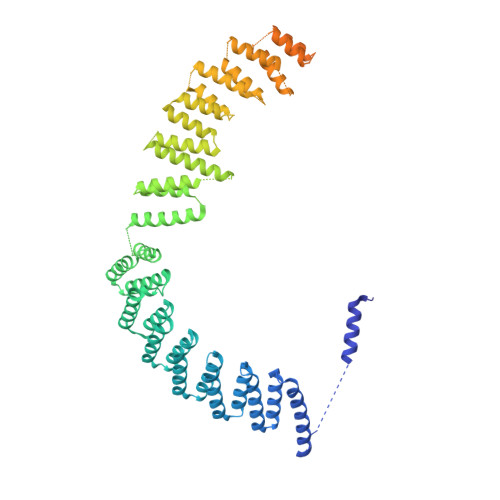
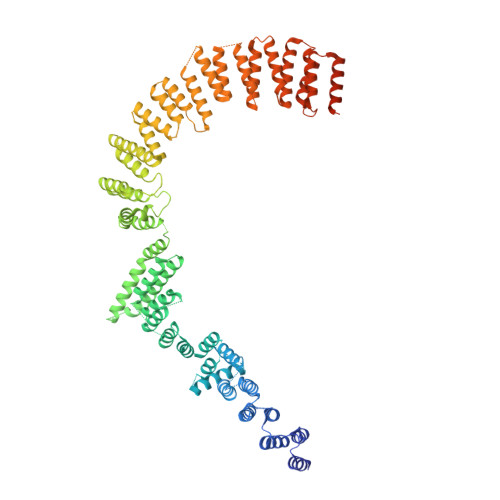
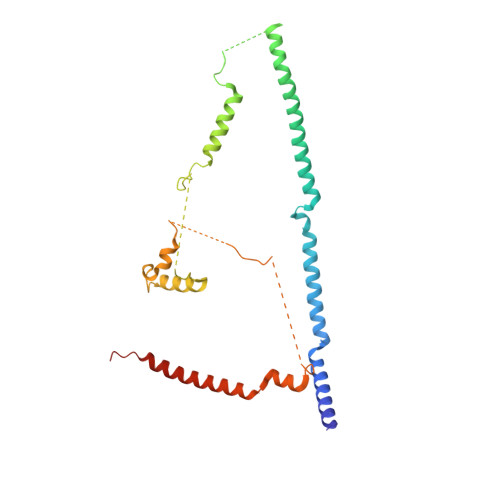
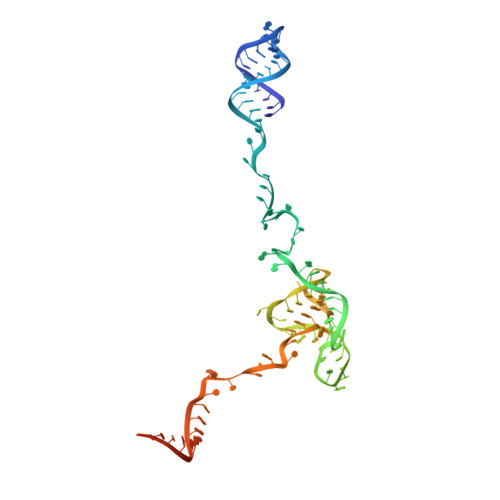
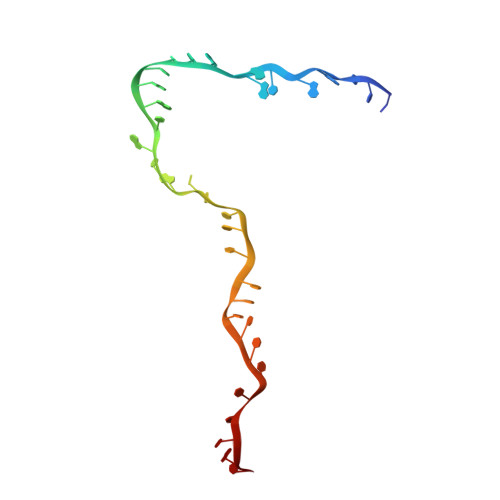
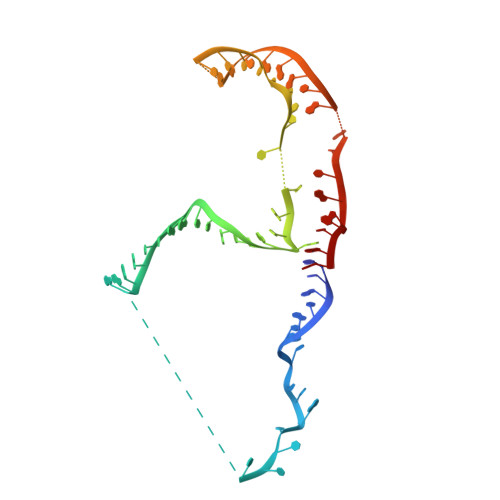
Structure of the yeast spliceosomal postcatalytic P complex.
Liu, S., Li, X., Zhang, L., Jiang, J., Hill, R.C., Cui, Y., Hansen, K.C., Zhou, Z.H., Zhao, R.(2017) Science 358: 1278-1283
- PubMed: 29146870
- DOI: https://doi.org/10.1126/science.aar3462
- Primary Citation of Related Structures:
6BK8 - PubMed Abstract:
The spliceosome undergoes dramatic changes in a splicing cycle. Structures of B, B act , C, C*, and intron lariat spliceosome complexes revealed mechanisms of 5'-splice site (ss) recognition, branching, and intron release, but lacked information on 3'-ss recognition, exon ligation, and exon release. Here we report a cryo-electron microscopy structure of the postcatalytic P complex at 3.3-angstrom resolution, revealing that the 3' ss is mainly recognized through non-Watson-Crick base pairing with the 5' ss and branch point. Furthermore, one or more unidentified proteins become stably associated with the P complex, securing the 3' exon and potentially regulating activity of the helicase Prp22. Prp22 binds nucleotides 15 to 21 in the 3' exon, enabling it to pull the intron-exon or ligated exons in a 3' to 5' direction to achieve 3'-ss proofreading or exon release, respectively.
- Electron Imaging Center for Nanomachines, University of California, Los Angeles (UCLA), Los Angeles, CA 90095, USA.
Organizational Affiliation: