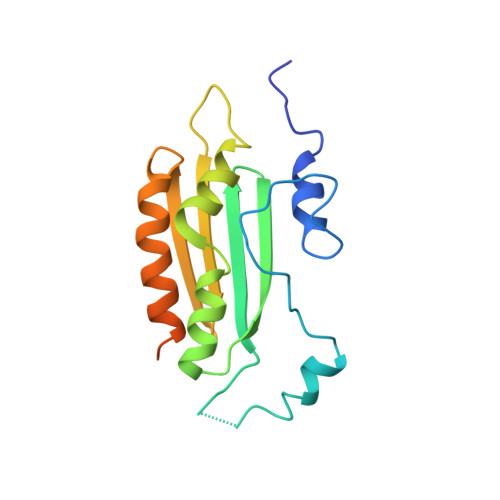
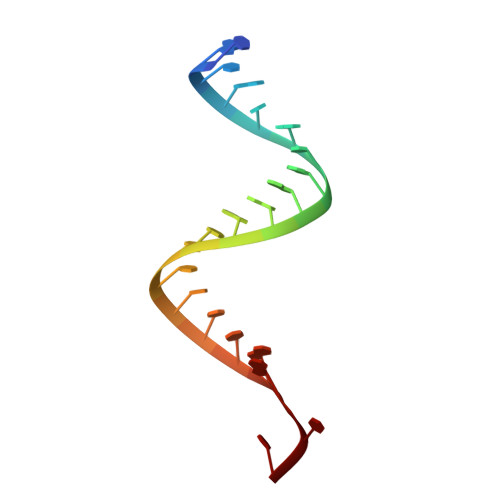
Structural insights into interactions between viral suppressor of RNA silencing protein p19 mutants and small RNAs.
Foss, D.V., Schirle, N.T., MacRae, I.J., Pezacki, J.P.(2019) FEBS Open Bio 9: 1042-1051
- PubMed: 31021526
- DOI: https://doi.org/10.1002/2211-5463.12644
- Primary Citation of Related Structures:
6BJG, 6BJH, 6BJV - PubMed Abstract:
Viral suppressors of RNA silencing (VSRSs) are a diverse group of viral proteins that have evolved to disrupt eukaryotic RNA silencing pathways, thereby contributing to viral pathogenicity. The p19 protein is a VSRS that selectively binds to short interfering RNAs (siRNAs) over microRNAs (miRNAs). Mutational analysis has identified single amino acid substitutions that reverse this selectivity through new high-affinity interactions with human miR-122. Herein, we report crystal structures of complexed p19-T111S (2.6 Å), p19-T111H (2.3 Å) and wild-type p19 protein (2.2 Å) from the Carnation Italian ringspot virus with small interfering RNA (siRNA) ligands. Structural comparisons reveal that these mutations do not lead to major changes in p19 architecture, but instead promote subtle rearrangement of residues and solvent molecules along the p19 midline. These observations suggest p19 uses many small interactions to distinguish siRNAs from miRNAs and perturbing these interactions can create p19 variants with novel RNA-recognition properties. DATABASE: Model data are deposited in the PDB database under the accession numbers 6BJG, 6BJH and 6BJV.
- Department of Biochemistry, Microbiology and Immunology, University of Ottawa, Canada.
Organizational Affiliation: