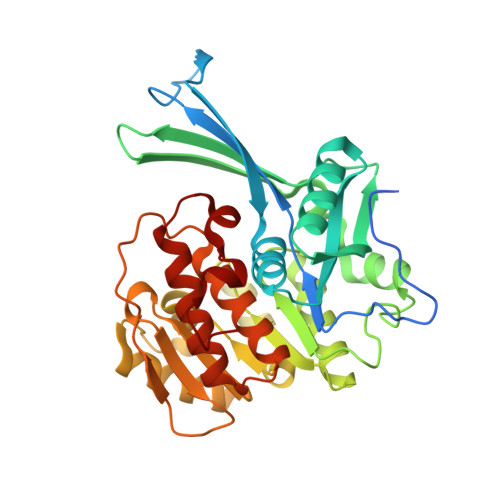
Unraveling structural insights of ribokinase from Leishmania donovani.
Gatreddi, S., Pillalamarri, V., Vasudevan, D., Addlagatta, A., Qureshi, I.A.(2019) Int J Biol Macromol 136: 253-265
- PubMed: 31170491
- DOI: https://doi.org/10.1016/j.ijbiomac.2019.06.001
- Primary Citation of Related Structures:
5ZWY, 6A8A, 6A8B, 6A8C - PubMed Abstract:
Ribokinase (RK) is an ATP dependent sugar kinase that enables the entry of ribose in the metabolism. Leishmania accumulates ribose into the cytosol through hydrolysis of nucleosides and by transport from the extracellular environment. Activation by RK is critical to mobilize the ribose into the metabolism of Leishmania. To understand the catalytic role, the crystal structure of RK (LdRK) from L. donovani was determined in the apo and complex forms with several nucleotides (ATP, AMPPCP and ADP) in the presence of Na + ion. The dual insertion of five amino acid stretches makes LdRK structurally unique from other reported structures of RKs. The structure of LdRK-ATP provided the basis for positioning of γ-phosphate of ATP by conserved -GAGD- motif. Liganded and unliganded structures of LdRK exists in similar conformation, which suggests binding of nucleotides does not make any significant conformational changes in nucleotide-bound structures. Substitution of a conserved asparagine with phenylalanine in ribose binding pocket differentiates the LdRK from other RKs. Glycerol molecule bound in the substrate binding pocket mimics the enzyme-substrate interactions but in turn, hampers the binding of ribose to LdRK. Comparative structural analysis revealed the flexibility of γ-phosphate, which adopts multiple conformations in the absence of divalent metal ion and ribose. Similar to other RKs, LdRK is also dependent on monovalent as well as divalent cations for its catalytic activity.
- Department of Biotechnology and Bioinformatics, School of Life Sciences, University of Hyderabad, Hyderabad 500 046, Telangana, India.
Organizational Affiliation: