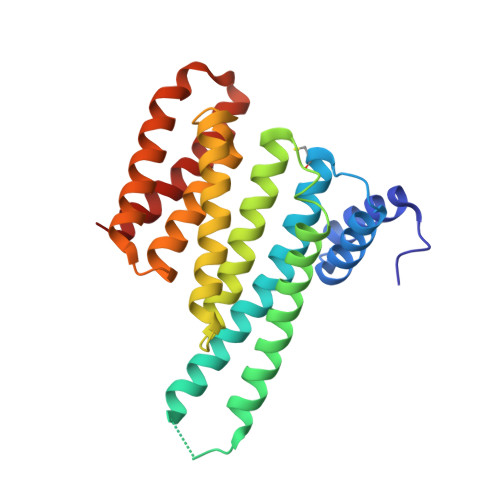
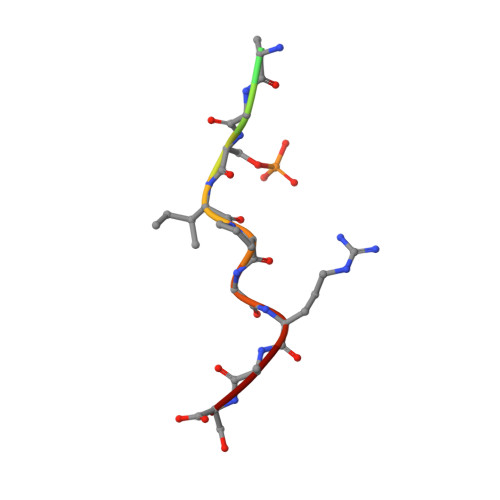
Fragment-Based Stabilizers of Protein-Protein Interactions through Imine-Based Tethering.
Wolter, M., Valenti, D., Cossar, P.J., Levy, L.M., Hristeva, S., Genski, T., Hoffmann, T., Brunsveld, L., Tzalis, D., Ottmann, C.(2020) Angew Chem Int Ed Engl 59: 21520-21524
- PubMed: 32816380
- DOI: https://doi.org/10.1002/anie.202008585
- Primary Citation of Related Structures:
6YOW, 6YOX, 6YOY, 6YP2, 6YP3, 6YP8, 6YPL, 6YPY, 6YQ2 - PubMed Abstract:
Small-molecule stabilization of protein-protein interactions (PPIs) is a promising concept in drug discovery, however the question how to identify or design chemical starting points in a "bottom-up" approach is largely unanswered. We report a novel concept for identifying initial chemical matter for PPI stabilization based on imine-forming fragments. The imine bond offers a covalent anchor for site-directed fragment targeting, whereas its transient nature enables efficient analysis of structure-activity relationships. This bond enables fragment identification and optimisation using protein crystallography. We report novel fragments that bind specifically to a lysine at the PPI interface of the p65-subunit-derived peptide of NF-κB with the adapter protein 14-3-3. Those fragments that subsequently establish contacts with the p65-derived peptide, rather than with 14-3-3, efficiently stabilize the 14-3-3/p65 complex and offer novel starting points for molecular glues.
- Laboratory of Chemical Biology, Department of Biomedical, Engineering and Institute for Complex Molecular Systems, Eindhoven University of Technology, P.O. Box 513, 5600 MB, Eindhoven, The Netherlands.
Organizational Affiliation: