Non-Coordinative Binding of O2 at the Active Center of a Copper-Dependent Enzyme
Leisinger, F., Miarzlou, D.A., Seebeck, F.P.(2020) Angew Chem Int Ed Engl
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
(2020) Angew Chem Int Ed Engl
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Formylglycine-generating enzyme | 303 | Thermomonospora curvata DSM 43183 | Mutation(s): 0 Gene Names: Tcur_4811 EC: 1.8.3.7 | 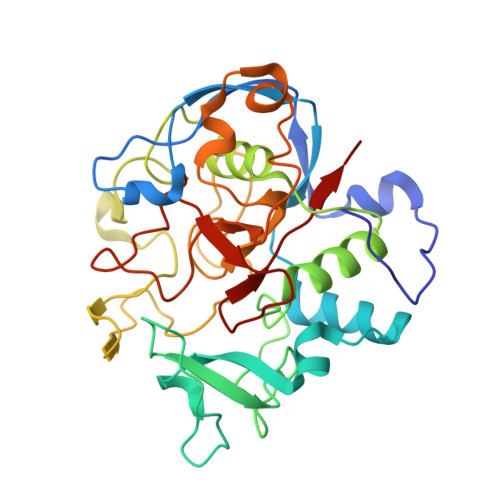 | |
UniProt | |||||
Find proteins for D1A7C3 (Thermomonospora curvata (strain ATCC 19995 / DSM 43183 / JCM 3096 / KCTC 9072 / NBRC 15933 / NCIMB 10081 / Henssen B9)) Explore D1A7C3 Go to UniProtKB: D1A7C3 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | D1A7C3 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| ABZ-ALA-THR-THR-PRO-LEU-CYS-GLY-PRO-SER-ARG-ALA-SER-ILE-LEU-SER-GLY | B [auth C] | 14 | Thermomonospora curvata DSM 43183 | Mutation(s): 0 | 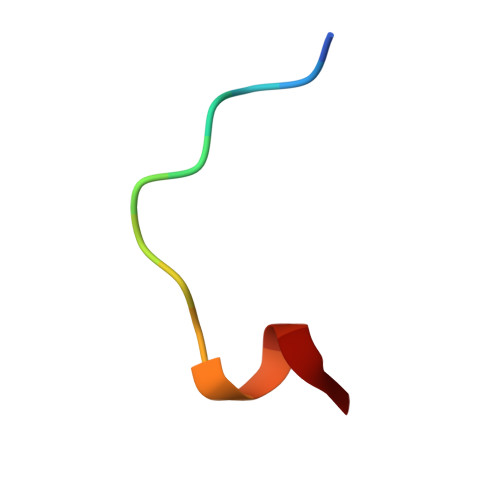 |
UniProt | |||||
Find proteins for D1ADF2 (Thermomonospora curvata (strain ATCC 19995 / DSM 43183 / JCM 3096 / KCTC 9072 / NBRC 15933 / NCIMB 10081 / Henssen B9)) Explore D1ADF2 Go to UniProtKB: D1ADF2 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | D1ADF2 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 4 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| SOA Query on SOA | G [auth C] | ISATOIC ANHYDRIDE C7 H9 N O VYFOAVADNIHPTR-UHFFFAOYSA-N |  | ||
| CU1 Query on CU1 | C [auth A] | COPPER (I) ION Cu VMQMZMRVKUZKQL-UHFFFAOYSA-N |  | ||
| CA Query on CA | D [auth A], E [auth A] | CALCIUM ION Ca BHPQYMZQTOCNFJ-UHFFFAOYSA-N |  | ||
| NO Query on NO | F [auth A] | NITRIC OXIDE N O ODUCDPQEXGNKDN-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 58.216 | α = 90 |
| b = 71.925 | β = 90 |
| c = 76.476 | γ = 90 |
| Software Name | Purpose |
|---|---|
| XDS | data reduction |
| Aimless | data scaling |
| PHENIX | refinement |
| PDB_EXTRACT | data extraction |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Swiss National Science Foundation | Switzerland | -- |
| European Research Council (ERC) | European Union | ERC-2013- StG 336559 |