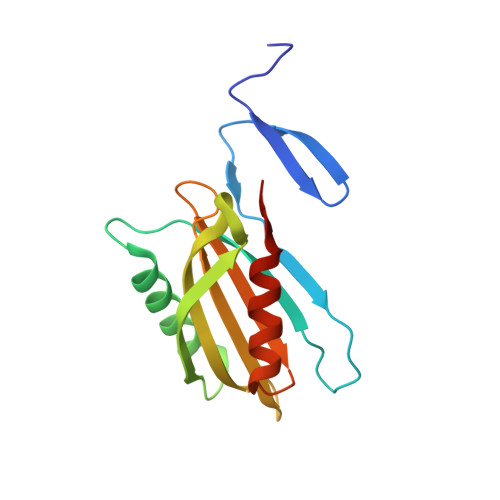
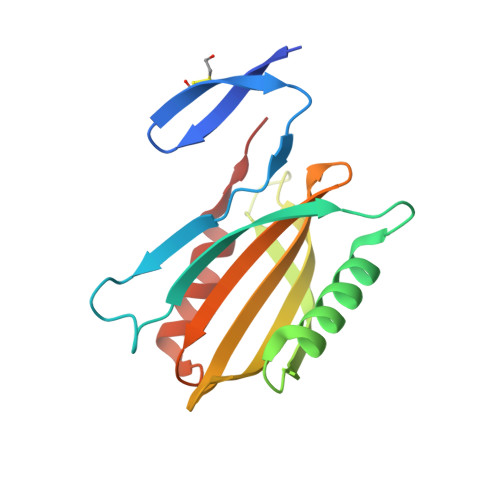
Structural basis for effector transmembrane domain recognition by type VI secretion system chaperones.
Ahmad, S., Tsang, K.K., Sachar, K., Quentin, D., Tashin, T.M., Bullen, N.P., Raunser, S., McArthur, A.G., Prehna, G., Whitney, J.C.(2020) Elife 9
- PubMed: 33320089
- DOI: https://doi.org/10.7554/eLife.62816
- Primary Citation of Related Structures:
6XRB, 6XRF, 6XRR - PubMed Abstract:
Type VI secretion systems (T6SSs) deliver antibacterial effector proteins between neighboring bacteria. Many effectors harbor N-terminal t rans m embrane d omains (TMDs) implicated in effector translocation across target cell membranes. However, the distribution of these TMD-containing effectors remains unknown. Here, we discover prePAAR, a conserved motif found in over 6000 putative TMD-containing effectors encoded predominantly by 15 genera of Proteobacteria. Based on differing numbers of TMDs, effectors group into two distinct classes that both require a member of the Eag family of T6SS chaperones for export. Co-crystal structures of class I and class II effector TMD-chaperone complexes from Salmonella Typhimurium and Pseudomonas aeruginosa , respectively, reveals that Eag chaperones mimic transmembrane helical packing to stabilize effector TMDs. In addition to participating in the chaperone-TMD interface, we find that prePAAR residues mediate effector-VgrG spike interactions. Taken together, our findings reveal mechanisms of chaperone-mediated stabilization and secretion of two distinct families of T6SS membrane protein effectors.
- Michael DeGroote Institute for Infectious Disease Research, McMaster University, Hamilton, Canada.
Organizational Affiliation: