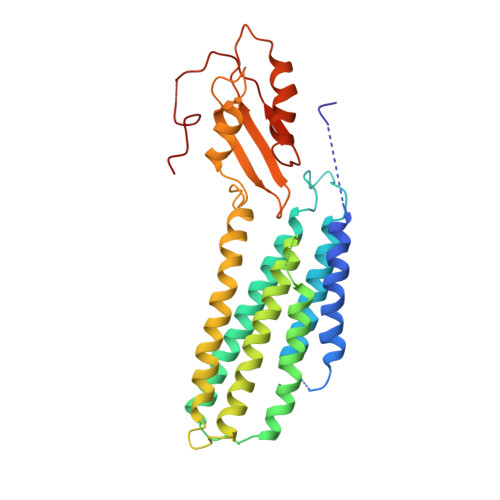
Cryo-EM structures of human ZnT8 in both outward- and inward-facing conformations.
Xue, J., Xie, T., Zeng, W., Jiang, Y., Bai, X.C.(2020) Elife 9
- PubMed: 32723473
- DOI: https://doi.org/10.7554/eLife.58823
- Primary Citation of Related Structures:
6XPD, 6XPE, 6XPF - PubMed Abstract:
ZnT8 is a Zn 2+ /H + antiporter that belongs to SLC30 family and plays an essential role in regulating Zn 2+ accumulation in the insulin secretory granules of pancreatic β cells. However, the Zn 2+ /H + exchange mechanism of ZnT8 remains unclear due to the lack of high-resolution structures. Here, we report the cryo-EM structures of human ZnT8 (HsZnT8) in both outward- and inward-facing conformations. HsZnT8 forms a dimeric structure with four Zn 2+ binding sites within each subunit: a highly conserved primary site in transmembrane domain (TMD) housing the Zn 2+ substrate; an interfacial site between TMD and C-terminal domain (CTD) that modulates the Zn 2+ transport activity of HsZnT8; and two adjacent sites buried in the cytosolic domain and chelated by conserved residues from CTD and the His-Cys-His (HCH) motif from the N-terminal segment of the neighboring subunit. A comparison of the outward- and inward-facing structures reveals that the TMD of each HsZnT8 subunit undergoes a large structural rearrangement, allowing for alternating access to the primary Zn 2+ site during the transport cycle. Collectively, our studies provide the structural insights into the Zn 2+ /H + exchange mechanism of HsZnT8.
- Howard Hughes Medical Institute and Department of Physiology, University of Texas Southwestern Medical Center, Dallas, United States.
Organizational Affiliation: