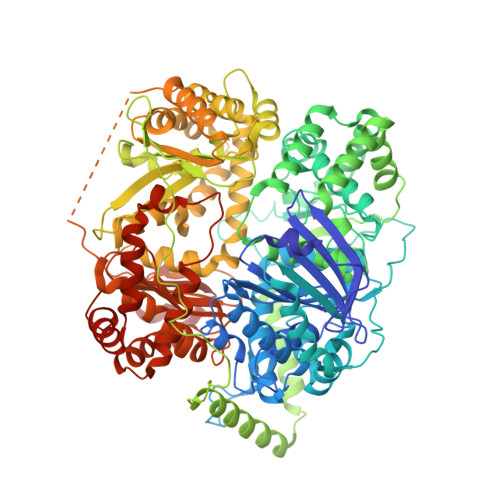
Structural basis for the mechanisms of human presequence protease conformational switch and substrate recognition.
Liang, W.G., Wijaya, J., Wei, H., Noble, A.J., Mancl, J.M., Mo, S., Lee, D., Lin King, J.V., Pan, M., Liu, C., Koehler, C.M., Zhao, M., Potter, C.S., Carragher, B., Li, S., Tang, W.J.(2022) Nat Commun 13: 1833-1833
- PubMed: 35383169
- DOI: https://doi.org/10.1038/s41467-022-29322-4
- Primary Citation of Related Structures:
6XOS, 6XOT, 6XOU, 6XOV - PubMed Abstract:
Presequence protease (PreP), a 117 kDa mitochondrial M16C metalloprotease vital for mitochondrial proteostasis, degrades presequence peptides cleaved off from nuclear-encoded proteins and other aggregation-prone peptides, such as amyloid β (Aβ). PreP structures have only been determined in a closed conformation; thus, the mechanisms of substrate binding and selectivity remain elusive. Here, we leverage advanced vitrification techniques to overcome the preferential denaturation of one of two ~55 kDa homologous domains of PreP caused by air-water interface adsorption. Thereby, we elucidate cryoEM structures of three apo-PreP open states along with Aβ- and citrate synthase presequence-bound PreP at 3.3-4.6 Å resolution. Together with integrative biophysical and pharmacological approaches, these structures reveal the key stages of the PreP catalytic cycle and how the binding of substrates or PreP inhibitor drives a rigid body motion of the protein for substrate binding and catalysis. Together, our studies provide key mechanistic insights into M16C metalloproteases for future therapeutic innovations.
- Ben-May Department for Cancer Research, The University of Chicago, Chicago, IL, USA.
Organizational Affiliation: