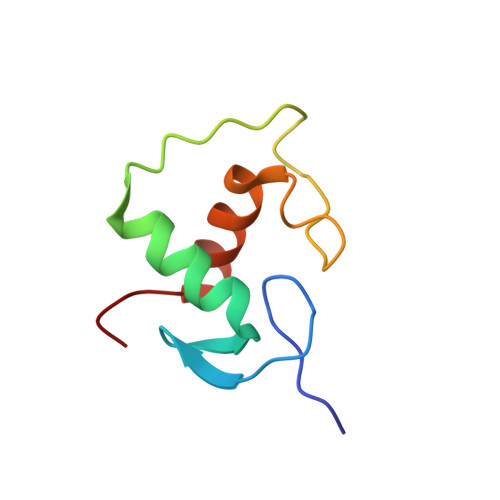
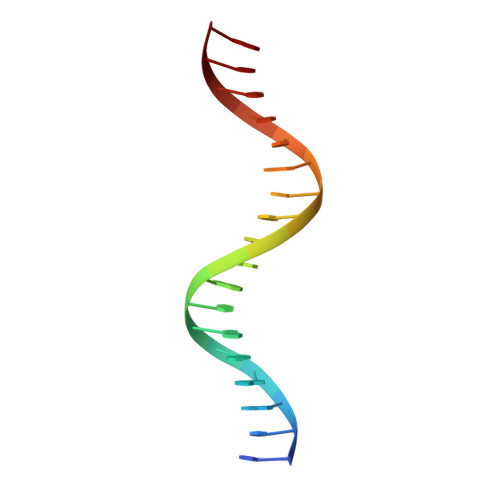
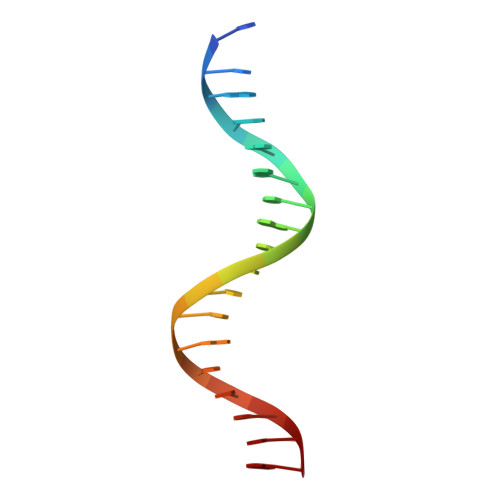
Structural basis for glucocorticoid receptor recognition of both unmodified and methylated binding sites, precursors of a modern recognition element.
Liu, X., Weikum, E.R., Tilo, D., Vinson, C., Ortlund, E.A.(2021) Nucleic Acids Res 49: 8923-8933
- PubMed: 34289059
- DOI: https://doi.org/10.1093/nar/gkab605
- Primary Citation of Related Structures:
6X6D, 6X6E - PubMed Abstract:
The most common form of DNA methylation involves the addition of a methyl group to a cytosine base in the context of a cytosine-phosphate-guanine (CpG) dinucleotide. Genomes from more primitive organisms are more abundant in CpG sites that, through the process of methylation, deamination and subsequent mutation to thymine-phosphate-guanine (TpG) sites, can produce new transcription factor binding sites. Here, we examined the evolutionary history of the over 36 000 glucocorticoid receptor (GR) consensus binding motifs in the human genome and identified a subset of them in regulatory regions that arose via a deamination and subsequent mutation event. GR can bind to both unmodified and methylated pre-GR binding sequences (GBSs) that contain a CpG site. Our structural analyses show that CpG methylation in a pre-GBS generates a favorable interaction with Arg447 mimicking that made with a TpG in a GBS. This methyl-specific recognition arose 420 million years ago and was conserved during the evolution of GR and likely helps fix the methylation on the relevant cytosines. Our study provides the first genetic, biochemical and structural evidence of high-affinity binding for the likely evolutionary precursor of extant TpG-containing GBS.
- Department of Biochemistry, Emory University School of Medicine, Atlanta, GA 30322, USA.
Organizational Affiliation: