Reconstitution of an intact clock reveals mechanisms of circadian timekeeping.
Chavan, A.G., Swan, J.A., Heisler, J., Sancar, C., Ernst, D.C., Fang, M., Palacios, J.G., Spangler, R.K., Bagshaw, C.R., Tripathi, S., Crosby, P., Golden, S.S., Partch, C.L., LiWang, A.(2021) Science 374: eabd4453-eabd4453
- PubMed: 34618577
- DOI: https://doi.org/10.1126/science.abd4453
- Primary Citation of Related Structures:
6X61 - PubMed Abstract:
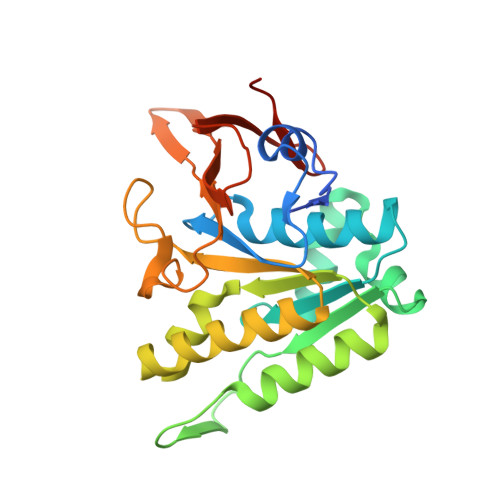
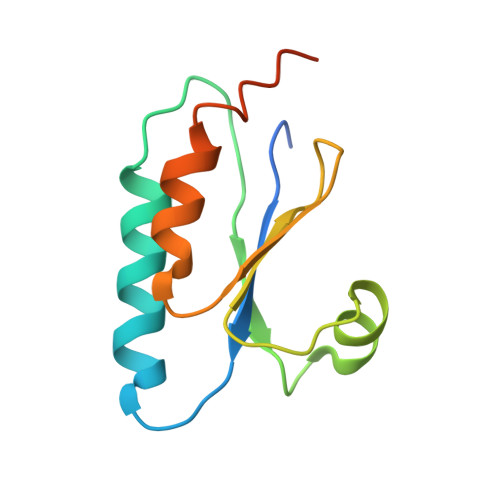
Circadian clocks control gene expression to provide an internal representation of local time. We report reconstitution of a complete cyanobacterial circadian clock in vitro, including the central oscillator, signal transduction pathways, downstream transcription factor, and promoter DNA. The entire system oscillates autonomously and remains phase coherent for many days with a fluorescence-based readout that enables real-time observation of each component simultaneously without user intervention. We identified the molecular basis for loss of cycling in an arrhythmic mutant and explored fundamental mechanisms of timekeeping in the cyanobacterial clock. We find that SasA, a circadian sensor histidine kinase associated with clock output, engages directly with KaiB on the KaiC hexamer to regulate period and amplitude of the central oscillator. SasA uses structural mimicry to cooperatively recruit the rare, fold-switched conformation of KaiB to the KaiC hexamer to form the nighttime repressive complex and enhance rhythmicity of the oscillator, particularly under limiting concentrations of KaiB. Thus, the expanded in vitro clock reveals previously unknown mechanisms by which the circadian system of cyanobacteria maintains the pace and rhythmicity under variable protein concentrations.
- School of Natural Sciences, University of California, Merced, CA 95343, USA.
Organizational Affiliation: