The crystal structure of AjiA1 reveals a novel structural motion mechanism in the adenylate-forming enzyme family
Paiva, F.C.R., Chan, K., Samborskyy, M., Silber, A.M., Leadlay, P., Dias, M.V.B.(2020) Acta Crystallogr D Biol Crystallogr 76: 1201
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
(2020) Acta Crystallogr D Biol Crystallogr 76: 1201
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| AjiA1 | 436 | Streptomyces sp. | Mutation(s): 0 | 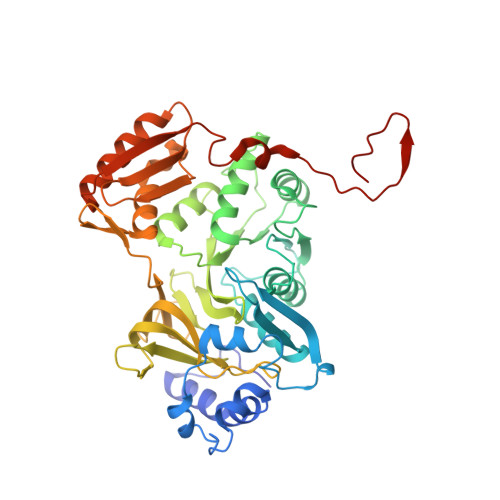 | |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ZN Query on ZN | C [auth A], F [auth B] | ZINC ION Zn PTFCDOFLOPIGGS-UHFFFAOYSA-N |  | ||
| MG Query on MG | D [auth A], E [auth A] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 128.777 | α = 90 |
| b = 128.777 | β = 90 |
| c = 101.449 | γ = 120 |
| Software Name | Purpose |
|---|---|
| Aimless | data scaling |
| PHENIX | refinement |
| PDB_EXTRACT | data extraction |
| XDS | data reduction |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Sao Paulo Research Foundation | Brazil | 2018/003511 |
| Brazilian National Council for Scientific and Technological Development | Brazil | 141090/2016-2 |