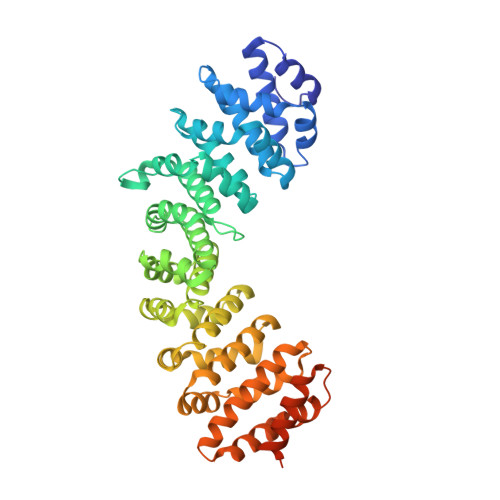
Structural and calorimetric studies reveal specific determinants for the binding of a high-affinity NLS to mammalian importin-alpha.
de Oliveira, H.C., da Silva, T.D., Salvador, G.H.M., Moraes, I.R., Fukuda, C.A., de Barros, A.C., Fontes, M.R.M.(2021) Biochem J 478: 2715-2732
- PubMed: 34195786
- DOI: https://doi.org/10.1042/BCJ20210401
- Primary Citation of Related Structures:
6WBA, 6WBB, 6WBC, 7M60 - PubMed Abstract:
The classical nuclear import pathway is mediated by importin (Impα and Impβ), which recognizes the cargo protein by its nuclear localization sequence (NLS). NLSs have been extensively studied resulting in different proposed consensus; however, recent studies showed that exceptions may occur. This mechanism may be also dependent on specific characteristics of different Impα. Aiming to better understand the importance of specific residues from consensus and adjacent regions of NLSs, we studied different mutations of a high-affinity NLS complexed to Impα by crystallography and calorimetry. We showed that although the consensus sequence allows Lys or Arg residues at the second residue of a monopartite sequence, the presence of Arg is very important to its binding in major and minor sites of Impα. Mutations in the N or C-terminus (position P1 or P6) of the NLS drastically reduces their affinity to the receptor, which is corroborated by the loss of hydrogen bonds and hydrophobic interactions. Surprisingly, a mutation in the far N-terminus of the NLS led to an increase in the affinity for both binding sites, corroborated by the structure with an additional hydrogen bond. The binding of NLSs to the human variant Impα1 revealed that these are similar to those found in structures presented here. For human variant Impα3, the bindings are only relevant for the major site. This study increases understanding of specific issues sparsely addressed in previous studies that are important to the task of predicting NLSs, which will be relevant in the eventual design of synthetic NLSs.
- Departamento de Biofísica e Farmacologia, Instituto de Biociências, Universidade Estadual Paulista, Botucatu, São Paulo, Brazil.
Organizational Affiliation: